Chapter 30: Nuclear Physics
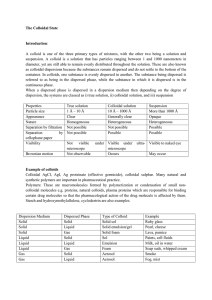
... Chemical elements are often written with the shorthand notation ZA X where A is the number of protons plus neutrons, Z is the number of protons, and X is the chemical symbol for the element. To find the binding energy of a nucleus, use binding energy B = ( Δm )c2 where Δm is the difference in mass ( ...
... Chemical elements are often written with the shorthand notation ZA X where A is the number of protons plus neutrons, Z is the number of protons, and X is the chemical symbol for the element. To find the binding energy of a nucleus, use binding energy B = ( Δm )c2 where Δm is the difference in mass ( ...
Molekylfysik - Leiden Institute of Physics
... If the energy E of the particle is below a finite barrier of potential V, the wavefunction of the particle is nonzero inside the barrier and outside the barrier. there is certain probability to find the particle outside the barrier, even though according to classical mechanics the particle has ins ...
... If the energy E of the particle is below a finite barrier of potential V, the wavefunction of the particle is nonzero inside the barrier and outside the barrier. there is certain probability to find the particle outside the barrier, even though according to classical mechanics the particle has ins ...
Atomic Structure, the Periodic Table, and Nuclear Radiation
... – The closer an electron is to the nucleus, the more strongly it is attracted. – The more protons in a nucleus, the more strongly an electron is attracted. 2. Electrons are repelled by other electrons in an atom. So, if other electrons are between a valence electron and the nucleus, the valence elec ...
... – The closer an electron is to the nucleus, the more strongly it is attracted. – The more protons in a nucleus, the more strongly an electron is attracted. 2. Electrons are repelled by other electrons in an atom. So, if other electrons are between a valence electron and the nucleus, the valence elec ...
What 3 ways can things become charged?
... In solid conductors, the electrons carry the charge through the circuit because they are loosely held. In fluids, like those in a car battery, positive and negative ions and electrons may compose the flow of electric charge. ...
... In solid conductors, the electrons carry the charge through the circuit because they are loosely held. In fluids, like those in a car battery, positive and negative ions and electrons may compose the flow of electric charge. ...
2012 - University of Utah Physics
... chain), which converts hydrogen nuclei (protons p) into helium nuclei (4 He). The overall reaction can be thought as that every four protons are converted into one 4 He nucleus, with an energy release Q. The masses of proton and 4 He nucleus are mp = 1.0076mu and m4 He = 4.0026mu , respectively, whe ...
... chain), which converts hydrogen nuclei (protons p) into helium nuclei (4 He). The overall reaction can be thought as that every four protons are converted into one 4 He nucleus, with an energy release Q. The masses of proton and 4 He nucleus are mp = 1.0076mu and m4 He = 4.0026mu , respectively, whe ...
Some basics of discrete space
... CP violation => One of the basic ingredient why we are here in the Universe , explain why there is more matter than anti-matter in our universe (Sahkarov, 1966). The discovery of the Kobayashi-Maskawa (1973) model of the Standard Model of electroweak interaction. Excellent place for the study of New ...
... CP violation => One of the basic ingredient why we are here in the Universe , explain why there is more matter than anti-matter in our universe (Sahkarov, 1966). The discovery of the Kobayashi-Maskawa (1973) model of the Standard Model of electroweak interaction. Excellent place for the study of New ...
- 1 - THE NATURE AND SPEED OF LIGHT Peter Kohut Maly Saris
... which the particle moves towards other objects. At the same time, the motions of external mutual connections between particles influence their internal motions. The various possible manifestations of a particle towards a magnetic field means, that the particle has various possibilities to perform it ...
... which the particle moves towards other objects. At the same time, the motions of external mutual connections between particles influence their internal motions. The various possible manifestations of a particle towards a magnetic field means, that the particle has various possibilities to perform it ...
Some Problems in String Cosmology
... CP violation => One of the basic ingredient why we are here in the Universe , explain why there is more matter than anti-matter in our universe (Sahkarov, 1966). The discovery of the Kobayashi-Maskawa (1973) model of the Standard Model of electroweak interaction. Excellent place for the study of New ...
... CP violation => One of the basic ingredient why we are here in the Universe , explain why there is more matter than anti-matter in our universe (Sahkarov, 1966). The discovery of the Kobayashi-Maskawa (1973) model of the Standard Model of electroweak interaction. Excellent place for the study of New ...
PWE 19-1: Magnetic Forces on a Proton and an Electron
... This example illustrates how the magnetic force on a moving charged particle depends on the direction in which the particle is moving. Note that the force magnitudes in parts (a), (b), and (c) are very small because a single electron or proton carries very little charge. These particles also have ve ...
... This example illustrates how the magnetic force on a moving charged particle depends on the direction in which the particle is moving. Note that the force magnitudes in parts (a), (b), and (c) are very small because a single electron or proton carries very little charge. These particles also have ve ...
Electrical Fields
... 6. a) Two point charges are located on x-axis (Figure 5.a). Determine the electric field exerted by +q at a point on a distance of d. Determine the electric force on Q. b) Thin rod of length 2L carry equal charges q uniformly distributed along their length. Determine the electric field exerted by t ...
... 6. a) Two point charges are located on x-axis (Figure 5.a). Determine the electric field exerted by +q at a point on a distance of d. Determine the electric force on Q. b) Thin rod of length 2L carry equal charges q uniformly distributed along their length. Determine the electric field exerted by t ...
Physics Today
... enough—specifically, when ∣β∣ ≫ ħ2/2μ. For n > 2, the classical approximation is valid only when the particles are close, approximately within one Bohr radius of each other. The short-range, exponential potentials are essentially beyond the reach of classical approaches. The ontological and epistemo ...
... enough—specifically, when ∣β∣ ≫ ħ2/2μ. For n > 2, the classical approximation is valid only when the particles are close, approximately within one Bohr radius of each other. The short-range, exponential potentials are essentially beyond the reach of classical approaches. The ontological and epistemo ...
File - Smile Of India
... The radiation was highly penetrating. The radiation was unaffected by magnetic and electric fields which show that it is electrically neutral. It was found to have approximately the same mass as the protons. The name ‘neutron’ was given to this sub-atomic particle. It is denoted by n or1on. Bombardm ...
... The radiation was highly penetrating. The radiation was unaffected by magnetic and electric fields which show that it is electrically neutral. It was found to have approximately the same mass as the protons. The name ‘neutron’ was given to this sub-atomic particle. It is denoted by n or1on. Bombardm ...
Optical Tests of Nanoengineered Liquid Mirrors
... liquid rotating in a gravitational field takes the shape of a parabola has been used to make large inexpensive parabolic mirrors. For a given diameter, the cost of a diffraction limited mercury liquid mirror is almost two orders of magnitudes lower than the cost of a glass mirror, making it very com ...
... liquid rotating in a gravitational field takes the shape of a parabola has been used to make large inexpensive parabolic mirrors. For a given diameter, the cost of a diffraction limited mercury liquid mirror is almost two orders of magnitudes lower than the cost of a glass mirror, making it very com ...
STATIC ELECTRICITY
... • If I double the distance the force decreases to one fourth of the original force • The Force between the spheres is directly proportional to the charge. ...
... • If I double the distance the force decreases to one fourth of the original force • The Force between the spheres is directly proportional to the charge. ...
Magnetic Force Exerted by a Magnetic Field on a Single Moving
... selector moving at speed v. ∑Fradial = m ac ...
... selector moving at speed v. ∑Fradial = m ac ...
Magnetic Force Exerted by a Magnetic Field on a Single Moving
... selector moving at speed v. ∑Fradial = m ac It then moves in a half ...
... selector moving at speed v. ∑Fradial = m ac It then moves in a half ...
Download PDF
... dislodge captured polystyrene beads under a given set of conditions. This flow has been compared to predictions made using models we have developed, and found to be in excellent agreement with experimental measurements with no fitted parameters. In addition, we have shown the utility of these modeli ...
... dislodge captured polystyrene beads under a given set of conditions. This flow has been compared to predictions made using models we have developed, and found to be in excellent agreement with experimental measurements with no fitted parameters. In addition, we have shown the utility of these modeli ...
III- Atomic Structure
... picture, i.e. an atom being largely empty space, it is easy to see why most α particles go right through the thin foil. when an α-particle happens to come near a nucleus the intense electric field there scatters it through a large angle, depending on the nuclear charge (i.e. atomic number) ...
... picture, i.e. an atom being largely empty space, it is easy to see why most α particles go right through the thin foil. when an α-particle happens to come near a nucleus the intense electric field there scatters it through a large angle, depending on the nuclear charge (i.e. atomic number) ...
Document
... the angular momentum ⇒ zero point energy can be zero! 3 Two wavefunctions with different quantum numbers can have the same energy. For example wavefunctions with ml = 1 and −1 have the same energy, ћ2/2I. This is known as degeneracy. Example: Let us treat the π-electrons in benzene as particles of m ...
... the angular momentum ⇒ zero point energy can be zero! 3 Two wavefunctions with different quantum numbers can have the same energy. For example wavefunctions with ml = 1 and −1 have the same energy, ћ2/2I. This is known as degeneracy. Example: Let us treat the π-electrons in benzene as particles of m ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.