August 2010 Regents Exam part 1
... 9 The percent composition by mass of nitrogen in NH4OH (gram-formula mass = 35 grams/mole) is equal to Nitrogen is 14 g out of 35 g molar mass ...
... 9 The percent composition by mass of nitrogen in NH4OH (gram-formula mass = 35 grams/mole) is equal to Nitrogen is 14 g out of 35 g molar mass ...
Homework No. 07 (Spring 2015) PHYS 530A: Quantum Mechanics II
... Table 1: Isospin assignments for particles. ...
... Table 1: Isospin assignments for particles. ...
Parallel Universes
... telescope to find further evidence. Radio Telescopes are used to find waves in space. If we can’t hear, see, or feel something in space, these tools could find waves for proof of something there. Even if it is just a planet, a black hole, or another dimension. ...
... telescope to find further evidence. Radio Telescopes are used to find waves in space. If we can’t hear, see, or feel something in space, these tools could find waves for proof of something there. Even if it is just a planet, a black hole, or another dimension. ...
Notes for lecture 7
... where h is the height of the particle above some fixed level. The PE is only defined up to an additive constant, because if the fixed level is chosen a distance h0 lower the PE will increase by mgh0 . 2 A more old-fashioned unit of power is the horsepower. There are various different definitions. The uni ...
... where h is the height of the particle above some fixed level. The PE is only defined up to an additive constant, because if the fixed level is chosen a distance h0 lower the PE will increase by mgh0 . 2 A more old-fashioned unit of power is the horsepower. There are various different definitions. The uni ...
pdf x1
... Electric forces exist between two charged particles The direction of electric force depends on the signs of the charges: forces between opposite sign charges are attractive forces between like sign g charges g are repulsive p ...
... Electric forces exist between two charged particles The direction of electric force depends on the signs of the charges: forces between opposite sign charges are attractive forces between like sign g charges g are repulsive p ...
Document
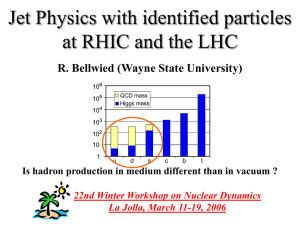
... compared to pp . Is this due to simple canonical suppression in pp ? Any predictions for charmed baryons ? Large associated particle yield in AA compared to pp. Long range Dh correlations might be due to recombination. There might be a baryon/meson trend in agreement with recombination, but it is a ...
... compared to pp . Is this due to simple canonical suppression in pp ? Any predictions for charmed baryons ? Large associated particle yield in AA compared to pp. Long range Dh correlations might be due to recombination. There might be a baryon/meson trend in agreement with recombination, but it is a ...
Graphene
... directional and energy dependence of transmission in graphene. • The problem #1 is manufacturing of clean samples. • Most of the physics observed so far is a single particle one. • Many-body effects are observed in FQHE in strong magnetic fields. The role of bending fluctuations is not very clear, t ...
... directional and energy dependence of transmission in graphene. • The problem #1 is manufacturing of clean samples. • Most of the physics observed so far is a single particle one. • Many-body effects are observed in FQHE in strong magnetic fields. The role of bending fluctuations is not very clear, t ...
8 Seeing the Heavens: Telescopes and Detectors
... 8.5 Non-Electromagnetic Observations Most, but not all, of our information in astronomy comes from detecting electromagnetic waves. Important examples of non-electromagnetic data include 1. Cosmic rays 2. Neutrinos 3. Gravitational waves ...
... 8.5 Non-Electromagnetic Observations Most, but not all, of our information in astronomy comes from detecting electromagnetic waves. Important examples of non-electromagnetic data include 1. Cosmic rays 2. Neutrinos 3. Gravitational waves ...
Potential Energy - McMaster Physics and Astronomy
... Elastic and Inelastic Collisions Momentum is conserved in collisions. Kinetic energy is sometimes conserved; it depends on the nature of the interaction force. A collision is called elastic if the total kinetic energy is the same before and after the collision. If the interaction force is conservat ...
... Elastic and Inelastic Collisions Momentum is conserved in collisions. Kinetic energy is sometimes conserved; it depends on the nature of the interaction force. A collision is called elastic if the total kinetic energy is the same before and after the collision. If the interaction force is conservat ...
Chapter 23 (Section 3) Pregnancy, Birth, and Childhood (Pages 735
... *e. There are currently 118 known ELEMENTS and 92 are found in nature, while the others are SYNTHETICALLY (man-made), but we only use between 30-40 elements daily *1. The discovery of all the ELEMENTS to date has taken THOUSANDS of years *2. In ancient times, it was believed there were “4” ELEMENTS: ...
... *e. There are currently 118 known ELEMENTS and 92 are found in nature, while the others are SYNTHETICALLY (man-made), but we only use between 30-40 elements daily *1. The discovery of all the ELEMENTS to date has taken THOUSANDS of years *2. In ancient times, it was believed there were “4” ELEMENTS: ...
Atoms and elements - Westmount High School
... francium has seven electron shells. As a result, its single valence electron is far from the nucleus and more loosely bound to it than the valence electrons of other alkali metals are bound to their nuclei. Consequently, francium reacts easily with other elements. ...
... francium has seven electron shells. As a result, its single valence electron is far from the nucleus and more loosely bound to it than the valence electrons of other alkali metals are bound to their nuclei. Consequently, francium reacts easily with other elements. ...
Chapter 33: The Atomic Nucleus and Radioactivity
... Ans. Alpha rays consist of positively charged helium nuclei. Beta rays consist of negatively charged electrons. Gamma rays are uncharged photons of light. A magnetic field will apply a force to a moving charged particle. Positively charged particles are accelerated in one direction and negative char ...
... Ans. Alpha rays consist of positively charged helium nuclei. Beta rays consist of negatively charged electrons. Gamma rays are uncharged photons of light. A magnetic field will apply a force to a moving charged particle. Positively charged particles are accelerated in one direction and negative char ...
Betatron - Atomic physics department
... keV at least to an energy of 10keV. Each magnetic flux tube is a trap since Bm>B0. Particle injection is impulsive, i.e. electrons fall into trap at the initial time and subsequently either precipitate into the loss cone or become trapped, acquiring additional energy. Due to motion from RR to chromo ...
... keV at least to an energy of 10keV. Each magnetic flux tube is a trap since Bm>B0. Particle injection is impulsive, i.e. electrons fall into trap at the initial time and subsequently either precipitate into the loss cone or become trapped, acquiring additional energy. Due to motion from RR to chromo ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.