Solutions
... 16. To probe the unknown charge of a nucleus, an alpha-particle of charge q = +2e, where e is the fundamental charge, and mass, m,, is launched directly toward it from far away with speed, v . The alpha-particle reaches a point of closest approach before turning around. Assume the unknown nucleus r ...
... 16. To probe the unknown charge of a nucleus, an alpha-particle of charge q = +2e, where e is the fundamental charge, and mass, m,, is launched directly toward it from far away with speed, v . The alpha-particle reaches a point of closest approach before turning around. Assume the unknown nucleus r ...
Charge to mass ratio of electron
... Energize the circuit to the coils, and bring the current up slowly. You should see the beam bend and then begin striking the top disk. Adjust the field current and plate potential until the beam strikes one of the etched circles (each circle will fluoresce when struck by the beam). You may need to a ...
... Energize the circuit to the coils, and bring the current up slowly. You should see the beam bend and then begin striking the top disk. Adjust the field current and plate potential until the beam strikes one of the etched circles (each circle will fluoresce when struck by the beam). You may need to a ...
Diapositive 1
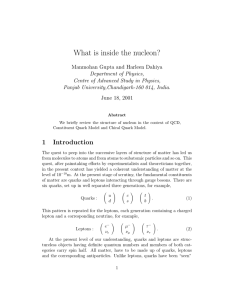
... In other words, the gauge-invariant extension of the gluon spin in light-cone gauge can be measured. Note that one can easily find gauge-invariant extensions of the gluon spin in other gauges. But we may not always find an experimental observable which reduces to the gluon spin in these gauges. [Hoo ...
... In other words, the gauge-invariant extension of the gluon spin in light-cone gauge can be measured. Note that one can easily find gauge-invariant extensions of the gluon spin in other gauges. But we may not always find an experimental observable which reduces to the gluon spin in these gauges. [Hoo ...
Atoms, Ions and Molecules The Building Blocks of Matter
... because most of the -particles passed through undeflected • The nucleus is very dense and positively charged because some of the -particles were repulsed and deflected • Electrons occupy the space around the nucleus • The atom is electrically neutral ...
... because most of the -particles passed through undeflected • The nucleus is very dense and positively charged because some of the -particles were repulsed and deflected • Electrons occupy the space around the nucleus • The atom is electrically neutral ...
PPT
... because most of the -particles passed through undeflected • The nucleus is very dense and positively charged because some of the -particles were repulsed and deflected • Electrons occupy the space around the nucleus • The atom is electrically neutral ...
... because most of the -particles passed through undeflected • The nucleus is very dense and positively charged because some of the -particles were repulsed and deflected • Electrons occupy the space around the nucleus • The atom is electrically neutral ...
The Electric Force
... Determine the initial launch velocity of the proton when it is very far from the deuterium by equating the work down by the electric force to the change in the proton’s kinetic energy. Assume the proton travels directly toward the deuterium and momentarily “stops” at the distance of closest approach ...
... Determine the initial launch velocity of the proton when it is very far from the deuterium by equating the work down by the electric force to the change in the proton’s kinetic energy. Assume the proton travels directly toward the deuterium and momentarily “stops” at the distance of closest approach ...
Lecture 5
... M-F 12:00AM -4:00PM. It is free. Hopefully all homework problems have been solved. Please see me immediately after the class if there is still an issue. ...
... M-F 12:00AM -4:00PM. It is free. Hopefully all homework problems have been solved. Please see me immediately after the class if there is still an issue. ...
Models of dark particle interactions with ordinary matter
... A particle cannot be considered as a point object in the localized region Comp. since part of the time it exists in the state “particle + the pair (particle-antiparticle)”. Compton wavelength does therefore determine the minimal uncertainty in the measurement of the particle’s coordinate. That is t ...
... A particle cannot be considered as a point object in the localized region Comp. since part of the time it exists in the state “particle + the pair (particle-antiparticle)”. Compton wavelength does therefore determine the minimal uncertainty in the measurement of the particle’s coordinate. That is t ...
SPH4U Modern Plans
... 1. Video from the Perimeter Institute – overview of modern physics. 2. Continue to work on the Photoelectric Effect Lab Unit 5 Day 6: Fun Physics 1. Class period to work on the last assignment in the computer lab. 2. Work on Fun Physics Assignment F1.1, F2.1, F3.1, F3.2 Text 12.4 – 12.5 Unit 5 Day 7 ...
... 1. Video from the Perimeter Institute – overview of modern physics. 2. Continue to work on the Photoelectric Effect Lab Unit 5 Day 6: Fun Physics 1. Class period to work on the last assignment in the computer lab. 2. Work on Fun Physics Assignment F1.1, F2.1, F3.1, F3.2 Text 12.4 – 12.5 Unit 5 Day 7 ...
Document
... If the collision is inelastic, kinetic energy of the system is not conserved, and additional information is probably needed If the collision is perfectly inelastic, the final velocities of the two objects are equal. Solve the momentum equations for the ...
... If the collision is inelastic, kinetic energy of the system is not conserved, and additional information is probably needed If the collision is perfectly inelastic, the final velocities of the two objects are equal. Solve the momentum equations for the ...
Electric Charge
... – Charges can be negative (like electrons) or positive (like protons). – In matter, the positive charges are stuck in place in the nuclei. Matter is negatively charged when extra electrons are added, and positively charged when electrons are removed. – Like charges repel, unlike charges attract. – C ...
... – Charges can be negative (like electrons) or positive (like protons). – In matter, the positive charges are stuck in place in the nuclei. Matter is negatively charged when extra electrons are added, and positively charged when electrons are removed. – Like charges repel, unlike charges attract. – C ...
e-over-m - Purdue Physics
... Early attempts to measure the mass of electrons proved futile. To address this issue, various direct experiments were devised. For instance, if a metal sphere of 1 meter radius is charged to a potential of -1×106 V, you can quickly estimate that about 7×1014 electrons must be added. Yet attempts to ...
... Early attempts to measure the mass of electrons proved futile. To address this issue, various direct experiments were devised. For instance, if a metal sphere of 1 meter radius is charged to a potential of -1×106 V, you can quickly estimate that about 7×1014 electrons must be added. Yet attempts to ...
fiitjee aieee class room program
... Two spherical bodies of mass M and 5M and radii R and 2R respectively are released in free space with initial separation between their centres equal to 12R. If they attract each other due to gravitational force only, then the distance covered by the smaller body just before collision is (A) 2.5R (B) ...
... Two spherical bodies of mass M and 5M and radii R and 2R respectively are released in free space with initial separation between their centres equal to 12R. If they attract each other due to gravitational force only, then the distance covered by the smaller body just before collision is (A) 2.5R (B) ...
Krishnendu-Sengupta
... Experiments with ultracold bosons on a lattice: finite rate dynamics 2D BEC confined in a trap and in the presence of an optical lattice. Single site imaging done by light-assisted collision which can reliably detect even/odd occupation of a site. In the present experiment one detects sites with n= ...
... Experiments with ultracold bosons on a lattice: finite rate dynamics 2D BEC confined in a trap and in the presence of an optical lattice. Single site imaging done by light-assisted collision which can reliably detect even/odd occupation of a site. In the present experiment one detects sites with n= ...
Elementary particle
In particle physics, an elementary particle or fundamental particle is a particle whose substructure is unknown, thus it is unknown whether it is composed of other particles. Known elementary particles include the fundamental fermions (quarks, leptons, antiquarks, and antileptons), which generally are ""matter particles"" and ""antimatter particles"", as well as the fundamental bosons (gauge bosons and Higgs boson), which generally are ""force particles"" that mediate interactions among fermions. A particle containing two or more elementary particles is a composite particle.Everyday matter is composed of atoms, once presumed to be matter's elementary particles—atom meaning ""indivisible"" in Greek—although the atom's existence remained controversial until about 1910, as some leading physicists regarded molecules as mathematical illusions, and matter as ultimately composed of energy. Soon, subatomic constituents of the atom were identified. As the 1930s opened, the electron and the proton had been observed, along with the photon, the particle of electromagnetic radiation. At that time, the recent advent of quantum mechanics was radically altering the conception of particles, as a single particle could seemingly span a field as would a wave, a paradox still eluding satisfactory explanation.Via quantum theory, protons and neutrons were found to contain quarks—up quarks and down quarks—now considered elementary particles. And within a molecule, the electron's three degrees of freedom (charge, spin, orbital) can separate via wavefunction into three quasiparticles (holon, spinon, orbiton). Yet a free electron—which, not orbiting an atomic nucleus, lacks orbital motion—appears unsplittable and remains regarded as an elementary particle.Around 1980, an elementary particle's status as indeed elementary—an ultimate constituent of substance—was mostly discarded for a more practical outlook, embodied in particle physics' Standard Model, science's most experimentally successful theory. Many elaborations upon and theories beyond the Standard Model, including the extremely popular supersymmetry, double the number of elementary particles by hypothesizing that each known particle associates with a ""shadow"" partner far more massive, although all such superpartners remain undiscovered. Meanwhile, an elementary boson mediating gravitation—the graviton—remains hypothetical.