Chapter 4 – Chemical Composition
... (a) The formula unit for an ionic compound is described by its formula, which shows the ratio of ions in lowest possible whole numbers. Since the formula of sodium chloride is NaCl, one formula unit is NaCl. From the image, you can see a one-to-one correspondence of sodium ions and chloride ions in ...
... (a) The formula unit for an ionic compound is described by its formula, which shows the ratio of ions in lowest possible whole numbers. Since the formula of sodium chloride is NaCl, one formula unit is NaCl. From the image, you can see a one-to-one correspondence of sodium ions and chloride ions in ...
Chapter 3 Solutions - Bremerton School District
... There are three peaks in the mass spectrum, each 2 mass units apart. This is consistent with two isotopes, differing in mass by two mass units. The peak at 157.84 corresponds to a Br2 molecule composed of two atoms of the lighter isotope. This isotope has mass equal to 157.84/2 or 78.92. This corres ...
... There are three peaks in the mass spectrum, each 2 mass units apart. This is consistent with two isotopes, differing in mass by two mass units. The peak at 157.84 corresponds to a Br2 molecule composed of two atoms of the lighter isotope. This isotope has mass equal to 157.84/2 or 78.92. This corres ...
Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]
... A combination reaction between a metal and a nonmetal, produces an ionic solid. Recall that the formula of an ionic compound can be determined from the charges of its ions When magnesium Mg2+ reacts with oxygen O2- , the magnesium loses electrons and forms the magnesium ion, Mg2+. The oxygen gains e ...
... A combination reaction between a metal and a nonmetal, produces an ionic solid. Recall that the formula of an ionic compound can be determined from the charges of its ions When magnesium Mg2+ reacts with oxygen O2- , the magnesium loses electrons and forms the magnesium ion, Mg2+. The oxygen gains e ...
Chapter 1 - Solutions
... molecular formula for ascorbic acid given that its molar mass is about 176 g. To find the empirical formula we need to know how many grams of C, H, and O there are in the sample. Since the sample only contains C, H, and O grams C + grams H + grams O = total mass We may use the results from the combu ...
... molecular formula for ascorbic acid given that its molar mass is about 176 g. To find the empirical formula we need to know how many grams of C, H, and O there are in the sample. Since the sample only contains C, H, and O grams C + grams H + grams O = total mass We may use the results from the combu ...
Stoichiometry: Calculations with Chemical Formulas and Equations
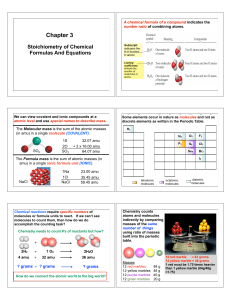
... • To calculate the limiting reactant 1) Convert to moles 2) Compare – pick 1 reactant, solve for the amount needed of the other and compare. 1.00 mol CO2 2 mol NaOH = 2.00 mol NaOH required (given = 1.70 mol) 1 mol CO2 or 1.70 mol NaOH 1 mol CO2 = 0.85 mol CO2 required 2 mol NaOH (given = 1.00 mol) ...
... • To calculate the limiting reactant 1) Convert to moles 2) Compare – pick 1 reactant, solve for the amount needed of the other and compare. 1.00 mol CO2 2 mol NaOH = 2.00 mol NaOH required (given = 1.70 mol) 1 mol CO2 or 1.70 mol NaOH 1 mol CO2 = 0.85 mol CO2 required 2 mol NaOH (given = 1.00 mol) ...
"Fundamentals of Rotation--Vibration Spectra" in
... functions. The resulting time-dependent dynamics of intramolecular energy flow is introduced as well. Effective Hamiltonians for interacting rotation–vibration levels are derived and applied to the practical treatment of complex spectra. Currently available computer programs aiding assignment and an ...
... functions. The resulting time-dependent dynamics of intramolecular energy flow is introduced as well. Effective Hamiltonians for interacting rotation–vibration levels are derived and applied to the practical treatment of complex spectra. Currently available computer programs aiding assignment and an ...
Chapter 3 2014
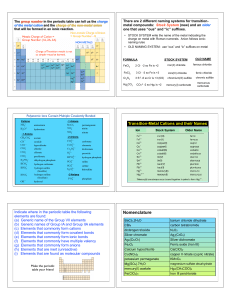
... Indicate where in the periodic table the following elements are found: (a) Generic name of the Group VII elements (b) Generic names of Group IA and Group IIA elements (c) Elements that commonly form cations (d) Elements that commonly form covalent bonds (e) Elements that commonly form ionic bonds (f ...
... Indicate where in the periodic table the following elements are found: (a) Generic name of the Group VII elements (b) Generic names of Group IA and Group IIA elements (c) Elements that commonly form cations (d) Elements that commonly form covalent bonds (e) Elements that commonly form ionic bonds (f ...
Chapter 3 2013
... Method 1: For both reactants, use balanced equation & stoichiometric factors and compute the amount of any product formed for both (I teach this method!) ...
... Method 1: For both reactants, use balanced equation & stoichiometric factors and compute the amount of any product formed for both (I teach this method!) ...
Slide 1
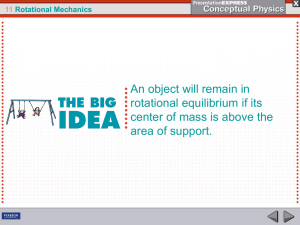
... 11.3 Center of Gravity Center of mass is often called center of gravity, the average position of all the particles of weight that make up an object. For almost all objects on and near Earth, these terms are interchangeable. There can be a small difference between center of gravity and center of mass ...
... 11.3 Center of Gravity Center of mass is often called center of gravity, the average position of all the particles of weight that make up an object. For almost all objects on and near Earth, these terms are interchangeable. There can be a small difference between center of gravity and center of mass ...
Slide 1
... rotational inertia depends on mass, but unlike inertia, rotational inertia depends on the distribution of the mass. The greater the distance between an object’s mass concentration and the axis of rotation, the greater the rotational inertia. ...
... rotational inertia depends on mass, but unlike inertia, rotational inertia depends on the distribution of the mass. The greater the distance between an object’s mass concentration and the axis of rotation, the greater the rotational inertia. ...
Chapter
... Representing Compounds Molecular Models • Models show the 3-dimensional structure along with all the other information given in structural formula • Ball-and-Stick Models use balls to represent the atoms and sticks to represent the ...
... Representing Compounds Molecular Models • Models show the 3-dimensional structure along with all the other information given in structural formula • Ball-and-Stick Models use balls to represent the atoms and sticks to represent the ...
Chapter
... Representing Compounds Molecular Models • Models show the 3-dimensional structure along with all the other information given in structural formula • Ball-and-Stick Models use balls to represent the atoms and sticks to represent the ...
... Representing Compounds Molecular Models • Models show the 3-dimensional structure along with all the other information given in structural formula • Ball-and-Stick Models use balls to represent the atoms and sticks to represent the ...
C H A P T E R
... 1. Count out exactly 200 small beads. Using a stopwatch, record the amount of time it takes you to count them. 2. Your teacher will tell you the approximate number of small beads in 1 g. Knowing that number, calculate the mass of 200 small beads. Record the mass that you have calculated. 3. Use a ba ...
... 1. Count out exactly 200 small beads. Using a stopwatch, record the amount of time it takes you to count them. 2. Your teacher will tell you the approximate number of small beads in 1 g. Knowing that number, calculate the mass of 200 small beads. Record the mass that you have calculated. 3. Use a ba ...
Chapter - Imperial Valley College
... Representing Compounds Molecular Models • Models show the 3-dimensional structure along with all the other information given in structural formula • Ball-and-Stick Models use balls to represent the atoms and sticks to represent the ...
... Representing Compounds Molecular Models • Models show the 3-dimensional structure along with all the other information given in structural formula • Ball-and-Stick Models use balls to represent the atoms and sticks to represent the ...
Chapter
... – use lines to represent covalent bonds – each line describes the number of electrons shared by the ...
... – use lines to represent covalent bonds – each line describes the number of electrons shared by the ...
Chemistry 11 - Correspondence Studies
... much product will be formed? This unit will answer these questions and other questions related to amount of matter. The word stoichiometry comes from the Greek words, stoicheion (meaning any first thing or principle) and metron (meaning measure). Stoichiometry deals with the mass-mass or molemole re ...
... much product will be formed? This unit will answer these questions and other questions related to amount of matter. The word stoichiometry comes from the Greek words, stoicheion (meaning any first thing or principle) and metron (meaning measure). Stoichiometry deals with the mass-mass or molemole re ...
QUANTUM MECHANICS B PHY-413 Note Set No. 7
... topic is quite long and complicated. I recommend that you first look at the summary section at the end, p.21, and then work through the material several times, looking through the summary at regular intervals. Your primary aim should be to understand the results given in the summary. The detailed der ...
... topic is quite long and complicated. I recommend that you first look at the summary section at the end, p.21, and then work through the material several times, looking through the summary at regular intervals. Your primary aim should be to understand the results given in the summary. The detailed der ...
Nonadiabatic alignment by intense pulses
... not experimentally) equivalent to the extensively studied problem of alignment of nonpolar molecules in a static (direct current, DC) electric field,23–30 recently discussed in the context of field-modified spectroscopy, e.g., in Refs. 31 and 32. Intense laser alignment in pump-probe experiments and ...
... not experimentally) equivalent to the extensively studied problem of alignment of nonpolar molecules in a static (direct current, DC) electric field,23–30 recently discussed in the context of field-modified spectroscopy, e.g., in Refs. 31 and 32. Intense laser alignment in pump-probe experiments and ...
Empirical and Molecular Formulas
... 54.24g of O X 1mol O/16.00g (atomic mass) of O= 3.390 mol of O Step 2: Determine simplest ratio by dividing the lowest amount of moles determined in step 1 3.390/3.390 = 1 mol of C 5.04/3.390 = 1.5 mol of H 3.390/3.390 = 1 mol of O Then look at the three numbers of moles and determine the lowest num ...
... 54.24g of O X 1mol O/16.00g (atomic mass) of O= 3.390 mol of O Step 2: Determine simplest ratio by dividing the lowest amount of moles determined in step 1 3.390/3.390 = 1 mol of C 5.04/3.390 = 1.5 mol of H 3.390/3.390 = 1 mol of O Then look at the three numbers of moles and determine the lowest num ...

![Stoichiometry Chapter 3 CHEMA1301 [Compatibility Mode]](http://s1.studyres.com/store/data/014247793_1-84b4b6fe6fa37d77afbf7eb657ee347a-300x300.png)