Monday, September 10 - Long Island University
... Quantum Mechanics • Key: Size of h sets the scale of what is small • If someone increased h, then at some points we would behave quantum mechanically • Birth of quantum mechanics because people couldn’t understand/explain: ...
... Quantum Mechanics • Key: Size of h sets the scale of what is small • If someone increased h, then at some points we would behave quantum mechanically • Birth of quantum mechanics because people couldn’t understand/explain: ...
spectral lines
... Emission and absorption spectra of gases like hydrogen indicate only certain wavelengths of light are emitted or absorbed by the atoms. ...
... Emission and absorption spectra of gases like hydrogen indicate only certain wavelengths of light are emitted or absorbed by the atoms. ...
3quarksdaily: More Is Different
... enumerate possibilities, and calculate the odds of them coming to pass. Nothing can be claimed for certain. This confusing world they had unwittingly ventured into, both fascinated and troubled the physicists of the time. They wondered where, in this crazy space, lay the innocent realm in which they ...
... enumerate possibilities, and calculate the odds of them coming to pass. Nothing can be claimed for certain. This confusing world they had unwittingly ventured into, both fascinated and troubled the physicists of the time. They wondered where, in this crazy space, lay the innocent realm in which they ...
Thesis Presentation Mr. Joshuah T. Heath Department of Physics
... analytical expression can be derived for the partition function at any density and chemical potential. In the canonical ensemble, the total number of particles, N, is fixed and an expression for the partition function can only be generated via a complicated recursion relation. In this work we apply ...
... analytical expression can be derived for the partition function at any density and chemical potential. In the canonical ensemble, the total number of particles, N, is fixed and an expression for the partition function can only be generated via a complicated recursion relation. In this work we apply ...
Problem set 4
... 900 Watts in a collimated beam in the x̂ direction. What is the force on the source? h2i 2. How many photons from a 100 MHz beam of FM radio waves must an electron absorb before it has gained an energy of 10 eV? h1i 3. Is the discreteness of the energy in an electromagnetic wave more easily detected ...
... 900 Watts in a collimated beam in the x̂ direction. What is the force on the source? h2i 2. How many photons from a 100 MHz beam of FM radio waves must an electron absorb before it has gained an energy of 10 eV? h1i 3. Is the discreteness of the energy in an electromagnetic wave more easily detected ...
Quantum Mechanics: Introduction
... but time periodicity of oscillator Additionally Laws of thermodynamics E = kT Fundamental constants : 1. velocity of light c 2. Avogadro Number N 3. Boltzman constant k 4. Unit of charge e ...
... but time periodicity of oscillator Additionally Laws of thermodynamics E = kT Fundamental constants : 1. velocity of light c 2. Avogadro Number N 3. Boltzman constant k 4. Unit of charge e ...
Heisenberg`s uncertainty principle
... quantum mechanics shows that certain pairs of physical properties, like position and speed, cannot both be known to arbitrary precision: the more precisely one property is known, the less precisely the other can be known. This statement is known as the uncertainty principle. The uncertainty principl ...
... quantum mechanics shows that certain pairs of physical properties, like position and speed, cannot both be known to arbitrary precision: the more precisely one property is known, the less precisely the other can be known. This statement is known as the uncertainty principle. The uncertainty principl ...
Erwin Schrodinger an Max Born and wavelength
... Erwin Schrodinger and wavelength mechanics • In 1926 he determined that a particle or an atom would vibrate in circles with activity • The atom contained, “Waves of Chance” • When an electron passed through the nucleus these waves would ripple back and forth • They would ripple in a straight line w ...
... Erwin Schrodinger and wavelength mechanics • In 1926 he determined that a particle or an atom would vibrate in circles with activity • The atom contained, “Waves of Chance” • When an electron passed through the nucleus these waves would ripple back and forth • They would ripple in a straight line w ...
Notes27and29January2014BasicQuantumMechanics
... Quantum Theory for Semiconductors How to determine the behavior of electrons in the semiconductor? • Mathematical description of motion of electrons in quantum mechanics ─ Schrödinger’s Equation • Solution of Schrödinger’s Equation energy band structure and probability of finding a electron at a pa ...
... Quantum Theory for Semiconductors How to determine the behavior of electrons in the semiconductor? • Mathematical description of motion of electrons in quantum mechanics ─ Schrödinger’s Equation • Solution of Schrödinger’s Equation energy band structure and probability of finding a electron at a pa ...
Atomic Diffraction Dr. Janine Shertzer College of the Holy Cross
... The wave-particle duality is fundamental to quantum mechanics. Light can behave like a particle (photon); matter can behave like a wave. The wavelength associated with a particle is inversely proportional to its momentum p: λ = h / p, where h is Planck’s constant. For cold atoms, the wavelength is l ...
... The wave-particle duality is fundamental to quantum mechanics. Light can behave like a particle (photon); matter can behave like a wave. The wavelength associated with a particle is inversely proportional to its momentum p: λ = h / p, where h is Planck’s constant. For cold atoms, the wavelength is l ...
PHYS6520 Quantum Mechanics II Spring 2013 HW #3
... Later on in this course we will compare this expression to the result of solving the Dirac equation in the presence of the Coulomb potential. (2) These questions are meant to associate numbers with atomic hydrogen phenomena. (a) The red n = 3 → 2 Balmer transition has a wavelength λ ≈ 656 nm. Calcul ...
... Later on in this course we will compare this expression to the result of solving the Dirac equation in the presence of the Coulomb potential. (2) These questions are meant to associate numbers with atomic hydrogen phenomena. (a) The red n = 3 → 2 Balmer transition has a wavelength λ ≈ 656 nm. Calcul ...
January 2009 - University of Michigan
... 8. (Atomic Physics) Basics of the level structure of the Helium atom. a) Sketch the energy level diagram for the lowest five (exactly five) energy levels of helium, ignoring fine structure splitting (i.e., show all levels for n = 1 and n = 2). Identify each level using spectroscopic notation. The l ...
... 8. (Atomic Physics) Basics of the level structure of the Helium atom. a) Sketch the energy level diagram for the lowest five (exactly five) energy levels of helium, ignoring fine structure splitting (i.e., show all levels for n = 1 and n = 2). Identify each level using spectroscopic notation. The l ...
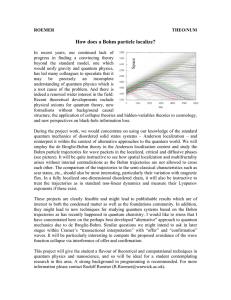
How does a Bohm particle localize?
... exponents if these exist. These projects are clearly feasible and might lead to publishable results which are of interest to both the condensed matter as well as the foundations community. In addition, they might lead to new techniques for studying quantum systems based on the Bohm trajectories as h ...
... exponents if these exist. These projects are clearly feasible and might lead to publishable results which are of interest to both the condensed matter as well as the foundations community. In addition, they might lead to new techniques for studying quantum systems based on the Bohm trajectories as h ...
PhD position: Quantum information processing with single electron spins
... PhD position: Quantum information processing with single electron spins in levitated diamonds A computer based on quantum information would be able to solve certain problems which are intractable with other types of computer. It is natural to use the spin of an electron as a quantum bit because spin ...
... PhD position: Quantum information processing with single electron spins in levitated diamonds A computer based on quantum information would be able to solve certain problems which are intractable with other types of computer. It is natural to use the spin of an electron as a quantum bit because spin ...