trt 408 physical chemistry
... arranged in patterns with long range, repeating order. Or Its may be amorphous in which case its atoms or molecules do not have any long range order. ...
... arranged in patterns with long range, repeating order. Or Its may be amorphous in which case its atoms or molecules do not have any long range order. ...
b. Artificial Neural Networks (ANN)
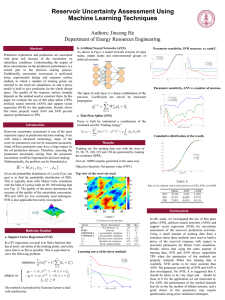
... selected to run reservoir simulations on and a proxy model is built to give prediction for the whole design space. The quality of the response surface strongly depends on the method used to construct them. In this paper we evaluate the use of thin plate spline (TPS), artificial neural network (ANN) ...
... selected to run reservoir simulations on and a proxy model is built to give prediction for the whole design space. The quality of the response surface strongly depends on the method used to construct them. In this paper we evaluate the use of thin plate spline (TPS), artificial neural network (ANN) ...
Document
... CHEMICAL EQUATION CHEMICAL EQUATION IS AN EASIER AND SHORTER WAY TO WRITE A CHEMICAL REACTION USING CHEMICAL SYMBOLS AND FORMULAS AS A SHORTCUT TO DESCRIBE A CHEMICAL REACTION ...
... CHEMICAL EQUATION CHEMICAL EQUATION IS AN EASIER AND SHORTER WAY TO WRITE A CHEMICAL REACTION USING CHEMICAL SYMBOLS AND FORMULAS AS A SHORTCUT TO DESCRIBE A CHEMICAL REACTION ...
AP CHEMISTRY – Source: 1999 AP Exam CHAPTER 8 TEST
... Glucose is polar and cyclohexane is nonpolar. • Polar solutes (such as glucose) are generally soluble in polar solvents such as water. • Nonpolar solutes (such as cyclohexane) are not soluble in the polar solvent. Students generally answered Part a of this question correctly. Students seemed to have ...
... Glucose is polar and cyclohexane is nonpolar. • Polar solutes (such as glucose) are generally soluble in polar solvents such as water. • Nonpolar solutes (such as cyclohexane) are not soluble in the polar solvent. Students generally answered Part a of this question correctly. Students seemed to have ...
Why Study Chemistry
... At the Particulate Level: “ Two atoms of carbon plus one diatomic molecule of oxygen yields two molecules of carbon monoxide “ Equation Coefficients: “ Gives the relative amount of each compound involved in the chemical equation “ Balanced Chemical Equations: “ The number of each kind of atom on the ...
... At the Particulate Level: “ Two atoms of carbon plus one diatomic molecule of oxygen yields two molecules of carbon monoxide “ Equation Coefficients: “ Gives the relative amount of each compound involved in the chemical equation “ Balanced Chemical Equations: “ The number of each kind of atom on the ...
The Analytical Study of Electronic and Optical Properties of Pyramid
... be noted that direct numerical calculations were used in these works, except, may be Ref. [19] where the analytical solution of Schrödinger equation for cone-shape restricted ...
... be noted that direct numerical calculations were used in these works, except, may be Ref. [19] where the analytical solution of Schrödinger equation for cone-shape restricted ...
NETADIS Research Project Overview The first list below gives the
... their electromagnetic fields modulated by a non-linear susceptibility. So far, localized mode distributions and nonlinear susceptibilities have never been successfully recovered from the analysis of the measurements of random laser spectra; this is a fundamental aim both conceptually (with the aim o ...
... their electromagnetic fields modulated by a non-linear susceptibility. So far, localized mode distributions and nonlinear susceptibilities have never been successfully recovered from the analysis of the measurements of random laser spectra; this is a fundamental aim both conceptually (with the aim o ...
CHEM 20 FINAL EXAM: STUDY HEADINGS Jan 2012
... Physical Chemistry: Solutions and Gases (Ch 14, 20, 21, 15, 18, 19) characteristics of the 3 phases of matter: solids, liquids, gases characteristics of phase changes: fusion, vaporization, liquefaction qualitative aspects of the kinetic theory: behavior of gases – pressure, volume Avogadro’s hypoth ...
... Physical Chemistry: Solutions and Gases (Ch 14, 20, 21, 15, 18, 19) characteristics of the 3 phases of matter: solids, liquids, gases characteristics of phase changes: fusion, vaporization, liquefaction qualitative aspects of the kinetic theory: behavior of gases – pressure, volume Avogadro’s hypoth ...
physical setting chemistry
... ideal gas based on the kinetic molecular theory? (1) The gas particles are relatively far apart and have negligible volume. (2) The gas particles are in constant, nonlinear motion. (3) The gas particles have attractive forces between them. (4) The gas particles have collisions without transferring e ...
... ideal gas based on the kinetic molecular theory? (1) The gas particles are relatively far apart and have negligible volume. (2) The gas particles are in constant, nonlinear motion. (3) The gas particles have attractive forces between them. (4) The gas particles have collisions without transferring e ...
ch5_slides
... So if hydrogen’s spectrum can tell us about the structure of a H atom, can the spectra of other elements help us create models of those atoms? http://jersey.uoregon.edu/vlab/elements/Elements.html What do we notice about the spectra of the other elements? They are more complex than H In general, th ...
... So if hydrogen’s spectrum can tell us about the structure of a H atom, can the spectra of other elements help us create models of those atoms? http://jersey.uoregon.edu/vlab/elements/Elements.html What do we notice about the spectra of the other elements? They are more complex than H In general, th ...
Chemical Stoichiometry
... an atomic mass unit (amu) is one twelfth the mass of a carbon-12 atom This gives us a basis for comparison The decimal numbers on the table are atomic masses in amu ...
... an atomic mass unit (amu) is one twelfth the mass of a carbon-12 atom This gives us a basis for comparison The decimal numbers on the table are atomic masses in amu ...
Chapter 3 Test Review
... formula (you must calculate) Step VI) Multiple the empirical formula by the number found in the step above. Remember this must be a whole number. ...
... formula (you must calculate) Step VI) Multiple the empirical formula by the number found in the step above. Remember this must be a whole number. ...
Unit 7 Packet
... NOTE: What is the relationship between synthesis and decomposition reactions? 3. Replacement (or single replacement – one element replaces another in a ...
... NOTE: What is the relationship between synthesis and decomposition reactions? 3. Replacement (or single replacement – one element replaces another in a ...
EXAM 3
... A 5.000 g sample of a compound known to contain only the elements phosphorous and oxygen was analyzed and found to contain 2.182 g of phosphorous. Additional experiments indicate that this compound has a molecular weight of 283.9 g/mol. How many phosphorous atoms are present in each molecule of this ...
... A 5.000 g sample of a compound known to contain only the elements phosphorous and oxygen was analyzed and found to contain 2.182 g of phosphorous. Additional experiments indicate that this compound has a molecular weight of 283.9 g/mol. How many phosphorous atoms are present in each molecule of this ...
Word Document
... 2. Explain the cause of spectral lines and why they are different for each element. 1. What Period 2 element has exactly three p orbital electrons in its shell? ...
... 2. Explain the cause of spectral lines and why they are different for each element. 1. What Period 2 element has exactly three p orbital electrons in its shell? ...
File
... Law of Conservation of Mass – Matter can neither be created nor destroyed but it can be converted from one form to another. o In ordinary reactions, no detectable changes in mass can be noted before and after the reaction. Law of Definite Proportion – A pure compound is always composed of the sa ...
... Law of Conservation of Mass – Matter can neither be created nor destroyed but it can be converted from one form to another. o In ordinary reactions, no detectable changes in mass can be noted before and after the reaction. Law of Definite Proportion – A pure compound is always composed of the sa ...
Chapter 3
... Solution For any value of the quantum number n, the allowed values of the quantum number l are integers ranging from 0 to (n − 1). For any value of the quantum number l, the allowed values of the quantum number ml are integers ranging from –l …0…+l. Apply these criteria to each case. (a) For n = 4, ...
... Solution For any value of the quantum number n, the allowed values of the quantum number l are integers ranging from 0 to (n − 1). For any value of the quantum number l, the allowed values of the quantum number ml are integers ranging from –l …0…+l. Apply these criteria to each case. (a) For n = 4, ...
Chemistry in Society - Cathkin High School
... more versatile than continuous as they can be used form ore than one reaction more suited for multi step reactions or when reaction time is long ...
... more versatile than continuous as they can be used form ore than one reaction more suited for multi step reactions or when reaction time is long ...