投影片 - 中正大學化生系
... chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of the atomic weight determines the character of the element, just as the magnitude of the molecule determines the character of a compound body. 5. We must expect the discovery of many yet u ...
... chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of the atomic weight determines the character of the element, just as the magnitude of the molecule determines the character of a compound body. 5. We must expect the discovery of many yet u ...
Tentative Chapter Three Assignments and Schedule
... Here is a list of quiz problems (partner, in-class, take-home or just "put them on the ch3 quiz" problems): 87,93 (if need more stoich),99 (if need more limiter), 107 (I usually give this one as a partner quiz...death by cyanide problem (kids like the name, but then they find it hard), but good revi ...
... Here is a list of quiz problems (partner, in-class, take-home or just "put them on the ch3 quiz" problems): 87,93 (if need more stoich),99 (if need more limiter), 107 (I usually give this one as a partner quiz...death by cyanide problem (kids like the name, but then they find it hard), but good revi ...
How many significant figures are there in each of these
... Matter is composed of small, chemically indivisible ATOMS ELEMENTS are kinds of matter that contain only a single kind of atom. All the atoms of an element have identical chemical properties. COMPOUNDS are kinds of matter that are composed of atoms of two or more ELEMENTS which are combined in simpl ...
... Matter is composed of small, chemically indivisible ATOMS ELEMENTS are kinds of matter that contain only a single kind of atom. All the atoms of an element have identical chemical properties. COMPOUNDS are kinds of matter that are composed of atoms of two or more ELEMENTS which are combined in simpl ...
matter crct/final exam review
... 26. All of the elements in a column are members of a _________________ and they all have the same number of _______________________________________________________. 27. What information does the atomic mass give you? 28. How can you calculate the number of neutrons in an atom? 29. The majority of th ...
... 26. All of the elements in a column are members of a _________________ and they all have the same number of _______________________________________________________. 27. What information does the atomic mass give you? 28. How can you calculate the number of neutrons in an atom? 29. The majority of th ...
standard sample test
... (c) The solution was found to be neither acidic nor basic, it was neutral. (d) The problem does not have enough information to determine if the solution was found to be acidic, basic or neutral. ...
... (c) The solution was found to be neither acidic nor basic, it was neutral. (d) The problem does not have enough information to determine if the solution was found to be acidic, basic or neutral. ...
Molecular Geometry and Chemical Bonding Theory
... valence bond (VB) theory (Linus Pauling) and molecular orbital (MO) theory (Robert S. Mulliken). The molecular orbital theory does a better job of describing molecules in their ...
... valence bond (VB) theory (Linus Pauling) and molecular orbital (MO) theory (Robert S. Mulliken). The molecular orbital theory does a better job of describing molecules in their ...
Exam 2 Form N - TAMU Chemistry
... a) Light has the characteristics of both a wave and a particle. b) The number of electrons ejected from a metal surface irradiated with visible light does not depend on the color of the light as long as the light is above a certain, minimum energy . c) Electrons in atoms are found in s, p, d, or f o ...
... a) Light has the characteristics of both a wave and a particle. b) The number of electrons ejected from a metal surface irradiated with visible light does not depend on the color of the light as long as the light is above a certain, minimum energy . c) Electrons in atoms are found in s, p, d, or f o ...
Optimal Conditioning of Quasi-Newton Methods
... of a scalar parameter. This paper derives necessary and sufficient conditions on the range of one such parameter to guarantee stability of the method. It further shows that the parameter effects only the length, not the direction, of the search vector at each step, and uses this result to derive sev ...
... of a scalar parameter. This paper derives necessary and sufficient conditions on the range of one such parameter to guarantee stability of the method. It further shows that the parameter effects only the length, not the direction, of the search vector at each step, and uses this result to derive sev ...
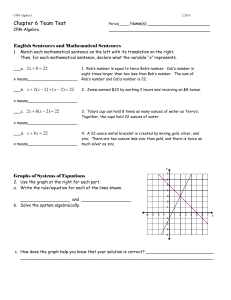
A variable needs to be eliminated to solve the system of equations
... your friend likes red marbles better than brown ones, the number of red marbles in the bag is 4 fewer than 5 times the number of brown marbles. The bag contains 74 marbles. a. Solve using a Guess & Check table. ...
... your friend likes red marbles better than brown ones, the number of red marbles in the bag is 4 fewer than 5 times the number of brown marbles. The bag contains 74 marbles. a. Solve using a Guess & Check table. ...
8872 Chemistry H1 syllabus for 2016
... technological world rather than focusing on large quantities of factual material which may have only short term relevance. Experimental work is an important component and should underpin the teaching and learning of Chemistry. ...
... technological world rather than focusing on large quantities of factual material which may have only short term relevance. Experimental work is an important component and should underpin the teaching and learning of Chemistry. ...
semester two final review key units 5 and 6 only
... 1. Definitions to know: monomer, polymer, biochemistry, hydrocarbon, carbohydrate, protein, lipids, and nucleic acids. Monomer: a molecule of any class of compounds, mostly organic that can react with other molecules that can form larger molecules Polymer: any of a class of natural or synthetic subs ...
... 1. Definitions to know: monomer, polymer, biochemistry, hydrocarbon, carbohydrate, protein, lipids, and nucleic acids. Monomer: a molecule of any class of compounds, mostly organic that can react with other molecules that can form larger molecules Polymer: any of a class of natural or synthetic subs ...
School of Chemistry and Physics Westville Campus, Durban
... Lewis structures are a simplified model that illustrates how valence electrons are shared between covalent compounds. ...
... Lewis structures are a simplified model that illustrates how valence electrons are shared between covalent compounds. ...
EXAM REVIEW !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!! The examination is scheduled
... reversible process, but is it applicable to any process within the restrictions of its derivation? What are the best (natural) thermodynamic variables for U? Be able to show how more Thermodynamic information comes from the definition of the exact differential and the exactness criterion. What are ...
... reversible process, but is it applicable to any process within the restrictions of its derivation? What are the best (natural) thermodynamic variables for U? Be able to show how more Thermodynamic information comes from the definition of the exact differential and the exactness criterion. What are ...
Role of Chemistry in Everyday Life
... prevention and treatment of diseases. If taken in doses higher than those recommended, most of the drugs used as medicines are potential poisons. Use of chemicals for therapeutic effect is called chemotherapy. February, 2016 |Journal of Chemistry and Chemical Sciences|www.chemistry-journal.org ...
... prevention and treatment of diseases. If taken in doses higher than those recommended, most of the drugs used as medicines are potential poisons. Use of chemicals for therapeutic effect is called chemotherapy. February, 2016 |Journal of Chemistry and Chemical Sciences|www.chemistry-journal.org ...
CH03_Tro_LectureNotes - Tutor
... A compound is made up of two or more elements or two or more types of atoms, chemically combined and therefore exists as molecules. Examples of compounds are water, H2O; sulfuric acid, H2SO4; carbon monoxide, CO.. Although there are two or more different types of atoms present, it is important to re ...
... A compound is made up of two or more elements or two or more types of atoms, chemically combined and therefore exists as molecules. Examples of compounds are water, H2O; sulfuric acid, H2SO4; carbon monoxide, CO.. Although there are two or more different types of atoms present, it is important to re ...
key to sample questions test 2
... t. Which of the following is the balanced net ionic equation for the precipitation of calcium phosphate when aqueous solutions of sodium phosphate and calcium chloride are mixed . 2Na3PO4(aq) + 3 CaCl2(aq) 6 NaCl(aq) + Ca3(PO4)2(s) 2 PO4(aq) + 3 Ca(aq) Ca3(PO4)2(s) PO4(aq) + Ca(aq) CaP ...
... t. Which of the following is the balanced net ionic equation for the precipitation of calcium phosphate when aqueous solutions of sodium phosphate and calcium chloride are mixed . 2Na3PO4(aq) + 3 CaCl2(aq) 6 NaCl(aq) + Ca3(PO4)2(s) 2 PO4(aq) + 3 Ca(aq) Ca3(PO4)2(s) PO4(aq) + Ca(aq) CaP ...
vsepr_lite_oct_2011 - chemistry11crescentsummer
... understand covalent bonding—polar and non-polar be able to draw Lewis structures for simple molecules and polyatomic ions, including molecules with double and triple bonds Introduction The premise of VSEPR theory: In a molecule or polyatomic ion, pairs of valence electrons on the central atom (c ...
... understand covalent bonding—polar and non-polar be able to draw Lewis structures for simple molecules and polyatomic ions, including molecules with double and triple bonds Introduction The premise of VSEPR theory: In a molecule or polyatomic ion, pairs of valence electrons on the central atom (c ...
Beyond the Lab: 25 Careers with a Chemistry Degree
... Moving Beyond the Lab: Part III • Graduate school is still a good idea in many careers • Other graduate programs to consider 1. Pharmacology‐chemistry of human body 2. Public Health/Epidemiology 3. Journalism/Science Writing 4. Specialty chemistry graduate programs 5. Programs in college of pha ...
... Moving Beyond the Lab: Part III • Graduate school is still a good idea in many careers • Other graduate programs to consider 1. Pharmacology‐chemistry of human body 2. Public Health/Epidemiology 3. Journalism/Science Writing 4. Specialty chemistry graduate programs 5. Programs in college of pha ...