First Year - WordPress.com
... Q. 28. A 50.00 mL sample of a cough mixture prepared by a pharmacist was found to have a mass of 46.0g. what is the density (in g/mL) of this mixture. Stated to the correct number of ...
... Q. 28. A 50.00 mL sample of a cough mixture prepared by a pharmacist was found to have a mass of 46.0g. what is the density (in g/mL) of this mixture. Stated to the correct number of ...
2. Essential Chemistry
... o Ratio of solute to solvent expressed as a percentage: weight (g)/volume (ml) o Unit seen on IV bags and medicinal solutions 5% dextrose = 5g dextrose / 100 ml of solution 0.9% saline = 0.9g NaCl / 100 ml of solution o Example: o Betadine antiseptic solution contains 10g of povidine-iodine in 1 ...
... o Ratio of solute to solvent expressed as a percentage: weight (g)/volume (ml) o Unit seen on IV bags and medicinal solutions 5% dextrose = 5g dextrose / 100 ml of solution 0.9% saline = 0.9g NaCl / 100 ml of solution o Example: o Betadine antiseptic solution contains 10g of povidine-iodine in 1 ...
APES Lesson 23B (2014-15) - Matter, Chemistry - science-b
... Law of the Conservation of Matter: The principle that matter many be transformed for one type of substance into another s, but it cannot be created or destroyed. Autotroph: An organism that produces its own food from inorganic compounds and a source of energy. There are photoautotrophs (photosynthet ...
... Law of the Conservation of Matter: The principle that matter many be transformed for one type of substance into another s, but it cannot be created or destroyed. Autotroph: An organism that produces its own food from inorganic compounds and a source of energy. There are photoautotrophs (photosynthet ...
Chemistry I Syllabus 2011-2012
... Weeks 5—10: Chapter 2 Fun with the Periodic Table, Active Chemistry Pages: 96 – 192 Essential Questions: 1. What specific properties of materials allow them to be classified as metals or nonmetals? 2. How is the relative mass of atoms determined? What does that indicate about the way in which they ...
... Weeks 5—10: Chapter 2 Fun with the Periodic Table, Active Chemistry Pages: 96 – 192 Essential Questions: 1. What specific properties of materials allow them to be classified as metals or nonmetals? 2. How is the relative mass of atoms determined? What does that indicate about the way in which they ...
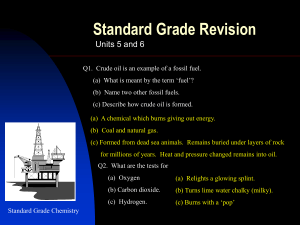
Unit 5 and 6 revsion - Deans Community High School
... Q14. The burning of fossil fuels is a major source of air pollution. (a) Name the acidic gas formed when air in a car engine is sparked. (b) Name the acidic gas formed when coal with a high sulphur content is burned. (c) Name the toxic gas formed when methane is burned in a limited supply of air. (d ...
... Q14. The burning of fossil fuels is a major source of air pollution. (a) Name the acidic gas formed when air in a car engine is sparked. (b) Name the acidic gas formed when coal with a high sulphur content is burned. (c) Name the toxic gas formed when methane is burned in a limited supply of air. (d ...
Chemistry 20
... It is designed for Academic Upgrading placement purposes only. This test may not be used for admission to any SAIT program; that is, this is not a SAIT admission exam. In addition, the results cannot be used at any other educational institution. The time allotted for the Chemistry 20 Placement test ...
... It is designed for Academic Upgrading placement purposes only. This test may not be used for admission to any SAIT program; that is, this is not a SAIT admission exam. In addition, the results cannot be used at any other educational institution. The time allotted for the Chemistry 20 Placement test ...
Introduction to the Chemistry of Life
... in this solution can be arranged in many more orientations with respect to each other than each salt solution alone. The set of dice with the 6’s showing on the side faces has more entropy since there are many more ways to obtain this configuration. The symmetric molecule has greater entropy, since ...
... in this solution can be arranged in many more orientations with respect to each other than each salt solution alone. The set of dice with the 6’s showing on the side faces has more entropy since there are many more ways to obtain this configuration. The symmetric molecule has greater entropy, since ...
rp oc4
... 5. Circle the lone pair electrons in the following dot formula of water. 6. With respect to bonds formed between the following pairs of atoms: • Determine the electronegativity difference. SHOW WORK! • Determine the probable bond type (ionic, polar covalent, or nonpolar covalent). • Assign partial ...
... 5. Circle the lone pair electrons in the following dot formula of water. 6. With respect to bonds formed between the following pairs of atoms: • Determine the electronegativity difference. SHOW WORK! • Determine the probable bond type (ionic, polar covalent, or nonpolar covalent). • Assign partial ...
What You Need to Know to Pass the Chemistry
... Nuclear fusion combines two light nuclei to form heavier nuclei. Nuclear fusion is the process that powers the sun. Nuclear fusion requires very high temperatures, and is not yet ready for practical use. The main advantage it offers is that the products are not radioactive wastes (as with fissio ...
... Nuclear fusion combines two light nuclei to form heavier nuclei. Nuclear fusion is the process that powers the sun. Nuclear fusion requires very high temperatures, and is not yet ready for practical use. The main advantage it offers is that the products are not radioactive wastes (as with fissio ...
Ch 8 Notes: Chemical Equations and Reactions
... Endothermic Reactions – when energy is absorbed or taken in during a chemical reaction. Energy is required when a compound is decomposed or breaks down; energy is a reactant and is written on the left of the arrow: 2H2O + energy 2H2 + O2 B. ...
... Endothermic Reactions – when energy is absorbed or taken in during a chemical reaction. Energy is required when a compound is decomposed or breaks down; energy is a reactant and is written on the left of the arrow: 2H2O + energy 2H2 + O2 B. ...
Document
... Two problems 1. Atomic masses do not convert easily to grams 2. They can’t be weighed (they are too small) ...
... Two problems 1. Atomic masses do not convert easily to grams 2. They can’t be weighed (they are too small) ...
Instructor`s Notes Atomic Tiles: Play Your Way from Atoms to
... 3a. Students know the structure of the atom and know it is composed of protons, neutrons, and electrons. 3b. Students know that compounds are formed by combining two or more different elements and that compounds have properties that are different from their constituent elements. 5a. Students know re ...
... 3a. Students know the structure of the atom and know it is composed of protons, neutrons, and electrons. 3b. Students know that compounds are formed by combining two or more different elements and that compounds have properties that are different from their constituent elements. 5a. Students know re ...
2002
... (b) By inspection, determine the spatial and temporal order of accuracy of scheme I (you do not need to derive the modified equation). (c) Perform von Neumann stability analyses for schemes I and II, and derive the amplification factors. (d) Experience shows that for large ∆t scheme I converges to t ...
... (b) By inspection, determine the spatial and temporal order of accuracy of scheme I (you do not need to derive the modified equation). (c) Perform von Neumann stability analyses for schemes I and II, and derive the amplification factors. (d) Experience shows that for large ∆t scheme I converges to t ...
CHAPTER 1, MATTER AND CHANGE Section 1, Chemistry Is a
... ! An element is a pure substance that cannot be broken down into simpler, stable substances and is made of one type of atom. (Example: hydrogen) ! A compound is a substance that can be broken down into simple stable substances. Each compound is made from the atoms of two or more elements that are ch ...
... ! An element is a pure substance that cannot be broken down into simpler, stable substances and is made of one type of atom. (Example: hydrogen) ! A compound is a substance that can be broken down into simple stable substances. Each compound is made from the atoms of two or more elements that are ch ...
2015 Academic Challenge CHEMISTRY TEST – STATE
... Academic Challenge sites will ask you to indicate your answer to each question by marking an oval that corresponds to the correct answer for that question. One oval should be marked to answer each question. Multiple ovals will automatically be graded as an incorrect answer. ...
... Academic Challenge sites will ask you to indicate your answer to each question by marking an oval that corresponds to the correct answer for that question. One oval should be marked to answer each question. Multiple ovals will automatically be graded as an incorrect answer. ...
Chapter 8
... formation of chemical bonds. • Electron pairs are localized between (shared or bonding pair) or on (lone pair) atoms such that each atom has an octet or duet of electrons. • VSEPR model predicts molecular geometry based on LE bonding model. ...
... formation of chemical bonds. • Electron pairs are localized between (shared or bonding pair) or on (lone pair) atoms such that each atom has an octet or duet of electrons. • VSEPR model predicts molecular geometry based on LE bonding model. ...
Fulltext PDF - Indian Academy of Sciences
... basis and then by single point PCM calculations at the same level but using the larger basis set. 2.3 Thermal energy and molecular entropy Thermal energy and entropy contribution towards the free energy change of the reductive process are obtained for the optimized geometry of a truncated model of t ...
... basis and then by single point PCM calculations at the same level but using the larger basis set. 2.3 Thermal energy and molecular entropy Thermal energy and entropy contribution towards the free energy change of the reductive process are obtained for the optimized geometry of a truncated model of t ...