Week 2
... - Compounds are formed when atoms of different elements unite in fixed proportions. That is, one atom of A to one of B in AB, two atoms of A to one of B in A2B and so on. - A chemical reaction involves a rearrangement of atoms to produce new compounds. No atoms are created, destroyed, or broken apar ...
... - Compounds are formed when atoms of different elements unite in fixed proportions. That is, one atom of A to one of B in AB, two atoms of A to one of B in A2B and so on. - A chemical reaction involves a rearrangement of atoms to produce new compounds. No atoms are created, destroyed, or broken apar ...
ch6 - ChemistryVCE
... a Metals and ionic solids both contain positive ions in a regular arrangement. b In metals and ionic solids, there is attraction between one particle and all the neighbouring particles of opposite charge. c In metals and ionic solids, there will be forces of repulsion between particles with like cha ...
... a Metals and ionic solids both contain positive ions in a regular arrangement. b In metals and ionic solids, there is attraction between one particle and all the neighbouring particles of opposite charge. c In metals and ionic solids, there will be forces of repulsion between particles with like cha ...
Ultracold Atoms in Line-World: Bose
... of matter predated those experiments by some 70 years [5]. From first principle, Bose derived the statistical behavior of photons. Upon receiving his draft, Einstein personally translated it into German to be published in the Zeitschrift für Physik [6], and then he extended the idea to matter [7]. ...
... of matter predated those experiments by some 70 years [5]. From first principle, Bose derived the statistical behavior of photons. Upon receiving his draft, Einstein personally translated it into German to be published in the Zeitschrift für Physik [6], and then he extended the idea to matter [7]. ...
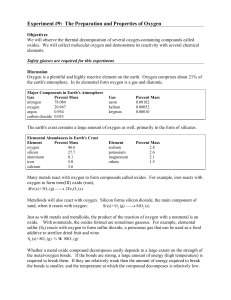
Experiment # 9 Properties of Oxygen
... the metal-oxygen bonds. If the bonds are strong, a large amount of energy (high temperature) is required to break them. If they are relatively weak then the amount of energy required to break the bonds is smaller, and the temperature at which the compound decomposes is relatively low. ...
... the metal-oxygen bonds. If the bonds are strong, a large amount of energy (high temperature) is required to break them. If they are relatively weak then the amount of energy required to break the bonds is smaller, and the temperature at which the compound decomposes is relatively low. ...
unit 7 h chem notes - chemical equations
... 1. Hydrogen gas reacts with chlorine gas to yield Hydrogen chloride. 2. Carbon reacts with oxygen gas to form Carbon dioxide. 3. Lithium reacts with chlorine gas to form Lithium Chloride. 4. Calcium reacts with Bromine to yield Calcium Bromide. 5. Hydrogen gas reacts with oxygen gas to yield water. ...
... 1. Hydrogen gas reacts with chlorine gas to yield Hydrogen chloride. 2. Carbon reacts with oxygen gas to form Carbon dioxide. 3. Lithium reacts with chlorine gas to form Lithium Chloride. 4. Calcium reacts with Bromine to yield Calcium Bromide. 5. Hydrogen gas reacts with oxygen gas to yield water. ...
Lecture 6
... First Law: A particle originally at rest, or moving in a straight line at constant velocity, will remain in this state if the resultant force acting on the particle is zero Second Law: If the resultant force on the particle is not zero, the particle experiences an acceleration in the same direction ...
... First Law: A particle originally at rest, or moving in a straight line at constant velocity, will remain in this state if the resultant force acting on the particle is zero Second Law: If the resultant force on the particle is not zero, the particle experiences an acceleration in the same direction ...
Worksheet 1 Notes - Department of Chemistry | Oregon State
... Determine the electron configuration for O. Is O a reactive element? Why? Determine the electron configuration for O-. Is O- a stable ion? Why? Determine the electron configuration for O2-. Is O2- a stable ion? Why? O is 1s22s22p4. O is very reactive (the principal quantum numbers 1 and 2 energy lev ...
... Determine the electron configuration for O. Is O a reactive element? Why? Determine the electron configuration for O-. Is O- a stable ion? Why? Determine the electron configuration for O2-. Is O2- a stable ion? Why? O is 1s22s22p4. O is very reactive (the principal quantum numbers 1 and 2 energy lev ...
CHAPTER 3
... 1. What is the average mass in grams of one avg. chlorine atom ? (5.89 X 10-23 g) 2. What is the avg. mass in grams of one ethanol (C2H5OH) molecule ? (7.65 X 10-23 g) 3. How many moles of PbCrO4 (Lead Chromate) are in 45.6 grams ? (0.141 mol) 4. How many HCl (hydrogen chloride) molecules are in 46. ...
... 1. What is the average mass in grams of one avg. chlorine atom ? (5.89 X 10-23 g) 2. What is the avg. mass in grams of one ethanol (C2H5OH) molecule ? (7.65 X 10-23 g) 3. How many moles of PbCrO4 (Lead Chromate) are in 45.6 grams ? (0.141 mol) 4. How many HCl (hydrogen chloride) molecules are in 46. ...
III. Quantum Model of the Atom
... A. Electrons as Waves Louis de Broglie (1924) Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
... A. Electrons as Waves Louis de Broglie (1924) Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
Di_AAAR_Poster - UNC
... with the known gas phase chemistry. Toluene, which is the most abundant of the aromatics, was chosen to be the study compound for kinetic model development. ...
... with the known gas phase chemistry. Toluene, which is the most abundant of the aromatics, was chosen to be the study compound for kinetic model development. ...
III. Quantum Model of the Atom
... A. Electrons as Waves Louis de Broglie (1924) Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
... A. Electrons as Waves Louis de Broglie (1924) Applied wave-particle theory to ee- exhibit wave properties QUANTIZED WAVELENGTHS ...
Lamb shift
... The spacetime curvature may cause corrections to quantum effects already existing in flat spacetime, e.g., the Lamb shift. The Lamb shift is weakened by the spacetime curvature, and the corrections may be found by looking at the spectra from a ...
... The spacetime curvature may cause corrections to quantum effects already existing in flat spacetime, e.g., the Lamb shift. The Lamb shift is weakened by the spacetime curvature, and the corrections may be found by looking at the spectra from a ...
ICCP Project 2 - Advanced Monte Carlo Methods
... physical quantities. The growth procedure starts with one atom and adds a second at a distance ...
... physical quantities. The growth procedure starts with one atom and adds a second at a distance ...
Class PPT - Madison Public Schools
... Early Theory of Electric Charge Physicists like Benjamin Franklin and Charles Coulomb believed that electric charge was a fluid. They theorized that when two objects are rubbed together, the fluid travels. Their theory also said that objects that have lots of the fluid are positively charged, and ob ...
... Early Theory of Electric Charge Physicists like Benjamin Franklin and Charles Coulomb believed that electric charge was a fluid. They theorized that when two objects are rubbed together, the fluid travels. Their theory also said that objects that have lots of the fluid are positively charged, and ob ...
78AM-1
... 5. A rod of length L and mass M is pivoted at one end to constitute a pendulum. Determine its period of oscillation and calculate its length if the period is desired to be 1 second. If the rod was instead suspended from a point at one quarter of its length, what would be the expression for the peri ...
... 5. A rod of length L and mass M is pivoted at one end to constitute a pendulum. Determine its period of oscillation and calculate its length if the period is desired to be 1 second. If the rod was instead suspended from a point at one quarter of its length, what would be the expression for the peri ...
Glossary: Chemical bonds
... mass units. The terms mass and weight are used interchangeably in this case. The atomic weight given on the periodic table is a weighted average of isotopic masses found in a typical terrestrial sample of the element. Atom. Compare with molecule and ion. An atom is the smallest particle of an elemen ...
... mass units. The terms mass and weight are used interchangeably in this case. The atomic weight given on the periodic table is a weighted average of isotopic masses found in a typical terrestrial sample of the element. Atom. Compare with molecule and ion. An atom is the smallest particle of an elemen ...
Empirical Formula
... • The molar mass is the mass in grams of one mole of a compound • The relative weights of molecules can be calculated from atomic masses water = H2O = 2(1.008 amu) + 16.00 amu = 18.02 amu • 1 mole of H2O will weigh 18.02 g, therefore the molar mass of H2O is 18.02 g • 1 mole of H2O will contain 16.0 ...
... • The molar mass is the mass in grams of one mole of a compound • The relative weights of molecules can be calculated from atomic masses water = H2O = 2(1.008 amu) + 16.00 amu = 18.02 amu • 1 mole of H2O will weigh 18.02 g, therefore the molar mass of H2O is 18.02 g • 1 mole of H2O will contain 16.0 ...
Charge and Mass of the Electron e me = 1.602×10−19 C 9.109×10
... (13) Latex Storage Bottle (14) HV Electrode Leads Fig. II-1: The Millikan Apparatus Theory The experiment is named for R. A. Millikan, the American physicist who devised it. (Millikan's original experiment used drops of oil, while this apparatus uses spheres of latex liquid.) Millikan wanted to dete ...
... (13) Latex Storage Bottle (14) HV Electrode Leads Fig. II-1: The Millikan Apparatus Theory The experiment is named for R. A. Millikan, the American physicist who devised it. (Millikan's original experiment used drops of oil, while this apparatus uses spheres of latex liquid.) Millikan wanted to dete ...
Chapter 3 – Stoichiometry of Formulas and Equations This chapter
... to determine a molecular formula is very important to chemists. Before we see how, it may have occurred to you that there is a bit of a problem with mass percent. Two compounds with the same ratio of atoms, will give the same mass percent. Formulas that contain the actual numbers of each type of ato ...
... to determine a molecular formula is very important to chemists. Before we see how, it may have occurred to you that there is a bit of a problem with mass percent. Two compounds with the same ratio of atoms, will give the same mass percent. Formulas that contain the actual numbers of each type of ato ...
Name - cloudfront.net
... How is the ideal gas law usually written? The combined gas law relates which items together? If a balloon is squeezed, what happens to the pressure of the gas inside the balloon? If the volume of a container of gas is reduced, what will happen to the pressure inside the container? In general, for a ...
... How is the ideal gas law usually written? The combined gas law relates which items together? If a balloon is squeezed, what happens to the pressure of the gas inside the balloon? If the volume of a container of gas is reduced, what will happen to the pressure inside the container? In general, for a ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.