Introduction: Proteins are one of the three major classes of biological
... Proteins perform an amazing array of different functions. This repertoire is enhanced when proteins bind to smaller chemicals called cofactors. You probably recognize some important protein-associated cofactors as vitamins. For example, vitamin B12 is a cobalt containing cofactor called cobalamin an ...
... Proteins perform an amazing array of different functions. This repertoire is enhanced when proteins bind to smaller chemicals called cofactors. You probably recognize some important protein-associated cofactors as vitamins. For example, vitamin B12 is a cobalt containing cofactor called cobalamin an ...
Document
... the ability of yeast extracts that lacked any living yeast cells to ferment sugar. In a series of experiments at the University of Berlin, he found that the sugar was fermented even when there were no living yeast cells in the mixture. He named the enzyme that brought about the fermentation of sucro ...
... the ability of yeast extracts that lacked any living yeast cells to ferment sugar. In a series of experiments at the University of Berlin, he found that the sugar was fermented even when there were no living yeast cells in the mixture. He named the enzyme that brought about the fermentation of sucro ...
B3. Enzymes - IGCSEBiology-Dnl
... due to low kinetic energy the collision frequency between enzyme and substrate is therefore low increasing the temperature, increases kinetic energy of molecules thus speeding up their movement, collision frequency between the substrates and the enzymes increases therefore enzyme activity increa ...
... due to low kinetic energy the collision frequency between enzyme and substrate is therefore low increasing the temperature, increases kinetic energy of molecules thus speeding up their movement, collision frequency between the substrates and the enzymes increases therefore enzyme activity increa ...
CHAPTER 6
... 1. To increase or decrease the number of enzyme molecule (enzyme level) 2. To increase or decrease the activity of each enzyme molecule (enzyme activity) ...
... 1. To increase or decrease the number of enzyme molecule (enzyme level) 2. To increase or decrease the activity of each enzyme molecule (enzyme activity) ...
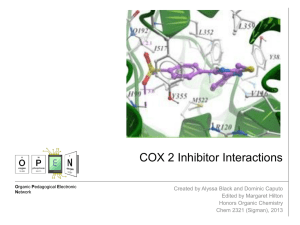
COX 2 Inhibitor Interactions - Center for Selective C–H
... Acetylation of COX-2 Active Site by Aspirin Aspirin can act as a COX-2 inhibitor, yet it is not as selective as most COX-2 inhibitors. Thus, it will also react with COX-1 enzymes, causing undesired side effects. When aspirin interacts with the COX-2 active site, it permanently acetylates a residue ...
... Acetylation of COX-2 Active Site by Aspirin Aspirin can act as a COX-2 inhibitor, yet it is not as selective as most COX-2 inhibitors. Thus, it will also react with COX-1 enzymes, causing undesired side effects. When aspirin interacts with the COX-2 active site, it permanently acetylates a residue ...
Formal Lab Report Guideline/Rubric
... enzyme activity. Changes in pH causes changes in hydrogen (H ) and hydroxyl (OH ) ions in solution. These changes in charge in the solution disrupt the secondary, tertiary and quaternary levels of structure of the proteins thereby changing the shape of the active site of the enzyme as the protein be ...
... enzyme activity. Changes in pH causes changes in hydrogen (H ) and hydroxyl (OH ) ions in solution. These changes in charge in the solution disrupt the secondary, tertiary and quaternary levels of structure of the proteins thereby changing the shape of the active site of the enzyme as the protein be ...
CHAPTERS 19 AND 20
... – The number of molecules of substrate acted on by one molecule of enzymes per minute Factors affecting enzyme activity – Enzyme concentration -increase of enzyme will increase the ES which increases the rate – Substrate concentration - increase of substrate at first increases rate until it reaches ...
... – The number of molecules of substrate acted on by one molecule of enzymes per minute Factors affecting enzyme activity – Enzyme concentration -increase of enzyme will increase the ES which increases the rate – Substrate concentration - increase of substrate at first increases rate until it reaches ...
Organic Chemistry
... according to the functions they perform. Structural proteins, for example help to form bones, muscles , shells, leaves, roots, and even the microscopic cell ‘skeleton’ that provides shape and allow ...
... according to the functions they perform. Structural proteins, for example help to form bones, muscles , shells, leaves, roots, and even the microscopic cell ‘skeleton’ that provides shape and allow ...
Chem*3560 Lecture 6: Allosteric regulation of enzymes
... induced by the another substance" (other than a substrate). R → T switch induced by binding CTP is called a negative heterotropic effect; and shifts the curve right. Negative because affinity decreases with increasing [CTP]. ATP and CTP are allosteric effectors, substances that do not participate di ...
... induced by the another substance" (other than a substrate). R → T switch induced by binding CTP is called a negative heterotropic effect; and shifts the curve right. Negative because affinity decreases with increasing [CTP]. ATP and CTP are allosteric effectors, substances that do not participate di ...
Chapter 4 - Open Yale Courses
... structure, the local domains are the secondary structure, the overall three-dimensional shape is the tertiary structure, and the formation of a complex with other polypeptide chains is the quaternary structure. • All the information necessary for a protein to fold properly into its tertiary structur ...
... structure, the local domains are the secondary structure, the overall three-dimensional shape is the tertiary structure, and the formation of a complex with other polypeptide chains is the quaternary structure. • All the information necessary for a protein to fold properly into its tertiary structur ...
BIOC*4540 ENZYMOLOGY Winter 2015
... to answer questions (independent study/seminar is worth 20% of the course grade). It is important to remember that anything that you include or say during your presentation is open to questions from the audience and so you should ensure that you fully understand it. Each student i ...
... to answer questions (independent study/seminar is worth 20% of the course grade). It is important to remember that anything that you include or say during your presentation is open to questions from the audience and so you should ensure that you fully understand it. Each student i ...
• What are enzymes? They`re special type of protein that accelerates
... changing its shape, it requires or acts on one molecule or one substrate • Lysases: addition or removal of one molecule to a chemical structure, without splitting it like adding to a double bond ...
... changing its shape, it requires or acts on one molecule or one substrate • Lysases: addition or removal of one molecule to a chemical structure, without splitting it like adding to a double bond ...
LEC15 EnzReg1 08
... conformational changes caused by binding of the same or other ligands at other sites on protein ("allosteric effects") • Changes involve simple association/dissociation of small molecules, so enzyme can cycle rapidly between active and inactive (or more and less active) states. 2. Interaction with r ...
... conformational changes caused by binding of the same or other ligands at other sites on protein ("allosteric effects") • Changes involve simple association/dissociation of small molecules, so enzyme can cycle rapidly between active and inactive (or more and less active) states. 2. Interaction with r ...
Camp 1 - Dr. Paul J. McElligott
... enzyme that must have part of its polypeptide chain cleaved before it becomes active • an example is trypsin, a digestive enzyme • it is synthesized and stored as trypsinogen, which has no enzyme activity • it becomes active only after a six-amino acid fragment is hydrolyzed from the N-terminal end ...
... enzyme that must have part of its polypeptide chain cleaved before it becomes active • an example is trypsin, a digestive enzyme • it is synthesized and stored as trypsinogen, which has no enzyme activity • it becomes active only after a six-amino acid fragment is hydrolyzed from the N-terminal end ...
1MBO Lopez kin
... investigation of both the microbial and eucaryotic enzyme, details of metal chelation remain unidentified. In this work we present the first structure of the wild-type human enzyme, a lead-inhibited intermediate of the wild-type enzyme with bound metallated porphyrin macrocycle, the product bound fo ...
... investigation of both the microbial and eucaryotic enzyme, details of metal chelation remain unidentified. In this work we present the first structure of the wild-type human enzyme, a lead-inhibited intermediate of the wild-type enzyme with bound metallated porphyrin macrocycle, the product bound fo ...
Cellular Energy and Enzymatic Function
... • Substrates bind to active site on enzyme • Binding induces conformational change in enzyme--better ”fit” for substrate • Active sites are highly specific and ...
... • Substrates bind to active site on enzyme • Binding induces conformational change in enzyme--better ”fit” for substrate • Active sites are highly specific and ...
Enzymes I
... activities of enzymes are determined by their three-dimensional structure. However, although structure does determine function, predicting a novel enzyme's activity just from its structure is a very difficult problem that has not yet been solved. Most enzymes are much larger than the substrates they ...
... activities of enzymes are determined by their three-dimensional structure. However, although structure does determine function, predicting a novel enzyme's activity just from its structure is a very difficult problem that has not yet been solved. Most enzymes are much larger than the substrates they ...
Chapter 2b Packet answers
... DNA (deoxyribonucleic acid)- genetic code, double stranded RNA (ribonucleic acid)- single stranded Function: Control’s cell’s activities, carries hereditary info, makes proteins E. ATP- energy for all cells (energy is in the bonds of the phosphate) Section 4 Enzymes- proteins that increase the speed ...
... DNA (deoxyribonucleic acid)- genetic code, double stranded RNA (ribonucleic acid)- single stranded Function: Control’s cell’s activities, carries hereditary info, makes proteins E. ATP- energy for all cells (energy is in the bonds of the phosphate) Section 4 Enzymes- proteins that increase the speed ...
Exam #3 2 Problem 1. (25 points) You study ligand binding to two
... molecule, but O2 and CO cannot simultaneously bind to the same heme. The binding affinity for CO is ~200 times higher than that for O2. Exposure for 1 hour to a CO concentration of 0.1% in inspired air leads to the occupancy by CO of about half the heme sites in Hb, a proportion that is frequently f ...
... molecule, but O2 and CO cannot simultaneously bind to the same heme. The binding affinity for CO is ~200 times higher than that for O2. Exposure for 1 hour to a CO concentration of 0.1% in inspired air leads to the occupancy by CO of about half the heme sites in Hb, a proportion that is frequently f ...
Enzymes - Capital High School
... reactant which binds to enzyme enzyme-substrate complex: temporary association ...
... reactant which binds to enzyme enzyme-substrate complex: temporary association ...
Multiple Choice
... B. testosterone C. ergosterol D. stigmasterol E. sodium cholate F. a cholestryl ester 14. Which of the following classes of lipids is not derived from arachidonic acid? A. prostaglandins B. thromboxanes C. leukotrienes D. isoprenoids E. All of the above are derived from arachidonic acid. 15. Transme ...
... B. testosterone C. ergosterol D. stigmasterol E. sodium cholate F. a cholestryl ester 14. Which of the following classes of lipids is not derived from arachidonic acid? A. prostaglandins B. thromboxanes C. leukotrienes D. isoprenoids E. All of the above are derived from arachidonic acid. 15. Transme ...
1 - u.arizona.edu
... depends on the ratio of the concentration of products to substrates in the cell; it is by manipulating this ratio that a cell can make an endergonic reaction proceed in the cell; this ability to change the free energy difference by altering the ratio of products to substrates is referred to as a mas ...
... depends on the ratio of the concentration of products to substrates in the cell; it is by manipulating this ratio that a cell can make an endergonic reaction proceed in the cell; this ability to change the free energy difference by altering the ratio of products to substrates is referred to as a mas ...
doc
... Competitive inhibition occurs when another substance, that has more affinity (attractive) power to the active site binds to the enzyme before the substrate can. This substance then prevents the enzyme from acting on the substrate and essentially stops its use for the time being. This can occur natur ...
... Competitive inhibition occurs when another substance, that has more affinity (attractive) power to the active site binds to the enzyme before the substrate can. This substance then prevents the enzyme from acting on the substrate and essentially stops its use for the time being. This can occur natur ...
study-guide-solutions-biochemistry
... the products are released. (c) Enzyme can catalyze another reaction. 4. Both cofactors and coenzymes are needed by some enzymes to carry out their catalytic activity. Cofactors are non-organic groups such as metals. Coenzymes are organic molecules such as vitamins. 5. (a) (b) ...
... the products are released. (c) Enzyme can catalyze another reaction. 4. Both cofactors and coenzymes are needed by some enzymes to carry out their catalytic activity. Cofactors are non-organic groups such as metals. Coenzymes are organic molecules such as vitamins. 5. (a) (b) ...
Enzyme inhibitor

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.