Enzymes - TeacherWeb
... • Proteins (ex: enzymes) are made up of DIFFERENT amino acids sequences (orders) • Each amino acid has different functional groups (R groups) • Different R groups in active site allow enzyme to bind different substrates ...
... • Proteins (ex: enzymes) are made up of DIFFERENT amino acids sequences (orders) • Each amino acid has different functional groups (R groups) • Different R groups in active site allow enzyme to bind different substrates ...
Amino acid metabolism III. Brake down of amino acids
... (Arg) • glutamine (Gln) • glutamate (Glu) • histidine (His) • proline (Pro) ...
... (Arg) • glutamine (Gln) • glutamate (Glu) • histidine (His) • proline (Pro) ...
enzymes - La Salle High School
... Lock and Key Model • An enzyme binds a substrate in a region called the active site • Only certain substrates can fit the active site • Amino acid R groups in the active site help substrate bind • Enzyme-substrate complex forms • Substrate reacts to form product ...
... Lock and Key Model • An enzyme binds a substrate in a region called the active site • Only certain substrates can fit the active site • Amino acid R groups in the active site help substrate bind • Enzyme-substrate complex forms • Substrate reacts to form product ...
Molecular Interactions in Cell events
... Trypsinogen is synthesised in the Pancreas Activation occurs when trypsinogen has amino acids removed in the duodenum by another protease enzyme This changes the trypsinogen into the active form trypsin Trypsin then helps to activate more trypsinogen molecules ...
... Trypsinogen is synthesised in the Pancreas Activation occurs when trypsinogen has amino acids removed in the duodenum by another protease enzyme This changes the trypsinogen into the active form trypsin Trypsin then helps to activate more trypsinogen molecules ...
Amino Acids, Proteins, and Enzymes
... Lock and Key Model • An enzyme binds a substrate in a region called the active site • Only certain substrates can fit the active site • Amino acid R groups in the active site help substrate bind • Enzyme-substrate complex forms • Substrate reacts to form product ...
... Lock and Key Model • An enzyme binds a substrate in a region called the active site • Only certain substrates can fit the active site • Amino acid R groups in the active site help substrate bind • Enzyme-substrate complex forms • Substrate reacts to form product ...
Enzymes - Michael P. Ready
... Common Tasks- Skill Level 1. You may self-administer the injection as follows: • Hold the injector in your hand forming a fist around the injector without covering or holding the needle end. • Place the end of the injector against your outer (lateral) thigh muscle anywhere from about a hand’s width ...
... Common Tasks- Skill Level 1. You may self-administer the injection as follows: • Hold the injector in your hand forming a fist around the injector without covering or holding the needle end. • Place the end of the injector against your outer (lateral) thigh muscle anywhere from about a hand’s width ...
The amino acids
... protein. Upon binding, two protons from the NH3 and one oxygen from the carboxyl join to form a water. So the peptide bond has at the one side a C=O and at the other side an N-H. Only the ends of the chain are NH3 or carboxylic. ...
... protein. Upon binding, two protons from the NH3 and one oxygen from the carboxyl join to form a water. So the peptide bond has at the one side a C=O and at the other side an N-H. Only the ends of the chain are NH3 or carboxylic. ...
Major Assignment: Modelling Carbohydrates, Lipids, and Proteins
... After all text questions, answer these questions under the heading “Additional Questions”. 1.) What is the function of glycogen? What is the function of cellulose? Explain why the molecular structures of each of these polysaccharides are well suited for their functions. (4 marks) 2.) Explain what 2 ...
... After all text questions, answer these questions under the heading “Additional Questions”. 1.) What is the function of glycogen? What is the function of cellulose? Explain why the molecular structures of each of these polysaccharides are well suited for their functions. (4 marks) 2.) Explain what 2 ...
Proteins - UF Macromolecular Structure Group
... The environment in the cell is crowded Protein interactions are highly specific (avoid non-productive interactions) Molecule that interacts with protein is called the LIGAND (or substrate) This can be another protein ...
... The environment in the cell is crowded Protein interactions are highly specific (avoid non-productive interactions) Molecule that interacts with protein is called the LIGAND (or substrate) This can be another protein ...
Micro Lab Unit 1 Flashcards
... 55) What is formed when oxygen accepts the H+ atoms? 56) Can enzymes be inhibited in many ways? 57) What are three ways that substances can act as an enzyme inhibitor? 58) An enzyme molecule is very large compared to its ______ and may contain several active sites. 59) What is a “false” substrate? 6 ...
... 55) What is formed when oxygen accepts the H+ atoms? 56) Can enzymes be inhibited in many ways? 57) What are three ways that substances can act as an enzyme inhibitor? 58) An enzyme molecule is very large compared to its ______ and may contain several active sites. 59) What is a “false” substrate? 6 ...
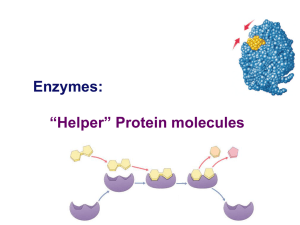
Enzymes: “Helper” Protein molecules
... Enzymes are not changed by the reaction used only temporarily – like a taxi re-used again for the same reaction with other molecules very little enzyme needed to help in many reactions ...
... Enzymes are not changed by the reaction used only temporarily – like a taxi re-used again for the same reaction with other molecules very little enzyme needed to help in many reactions ...
103 Lecture Ch21a
... • Isoenzymes are different forms of an enzyme that catalyze the same reaction in different tissues in the body - they have slight variations in the amino acid sequences of the subunits of their quaternary structure • For example, lactate dehydrogenase (LDH), which converts lactate to pyruvate, consi ...
... • Isoenzymes are different forms of an enzyme that catalyze the same reaction in different tissues in the body - they have slight variations in the amino acid sequences of the subunits of their quaternary structure • For example, lactate dehydrogenase (LDH), which converts lactate to pyruvate, consi ...
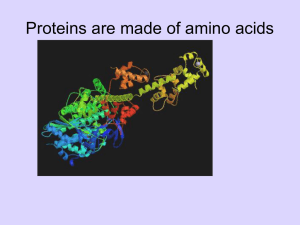
4.1_Proteins_Amino_Acids_2011
... chain. The peptide bond is planar (gray shading) and does not permit rotation. By contrast, rotation can occur about the Cα–C bond, whose angle of rotation is called psi (ψ), and about the N–Cα bond, whose angle of rotation is called phi (ϕ). By convention, an R group is often used to denote an amin ...
... chain. The peptide bond is planar (gray shading) and does not permit rotation. By contrast, rotation can occur about the Cα–C bond, whose angle of rotation is called psi (ψ), and about the N–Cα bond, whose angle of rotation is called phi (ϕ). By convention, an R group is often used to denote an amin ...
Enzymes
... place in cells. 2. Enzymes are very specific, generally catalyzing only one chemical reaction. 3. For this reason, part of an enzyme’s name is usually derived from the reaction it catalyzes. Enzymes usually end in the suffix “–ase”. Ex. Alcohol dehydrogenase catalyzes the reaction that removes water ...
... place in cells. 2. Enzymes are very specific, generally catalyzing only one chemical reaction. 3. For this reason, part of an enzyme’s name is usually derived from the reaction it catalyzes. Enzymes usually end in the suffix “–ase”. Ex. Alcohol dehydrogenase catalyzes the reaction that removes water ...
2-7 Active-Site Geometry
... molecules combine, both of them must collide reactive side-to-reactive side. Any other orientation and the collision will be non-productive. Thus, if both molecules first bind to an enzyme active site, and do so in such a way that their reactive portions are juxtaposed, the probability of a reaction ...
... molecules combine, both of them must collide reactive side-to-reactive side. Any other orientation and the collision will be non-productive. Thus, if both molecules first bind to an enzyme active site, and do so in such a way that their reactive portions are juxtaposed, the probability of a reaction ...
Amino acids catabolism
... The conversion of serine to glycine involves one-C unit from serine to an acceptor This is catalyzed by serine hydroxymethylase, with pyridoxal phosphate as coenzyme The acceptor is tetrahydropholate (derivative of folic acid) – its structure has 3 parts: a subtituted pteridine ring, p-aminobenzoic ...
... The conversion of serine to glycine involves one-C unit from serine to an acceptor This is catalyzed by serine hydroxymethylase, with pyridoxal phosphate as coenzyme The acceptor is tetrahydropholate (derivative of folic acid) – its structure has 3 parts: a subtituted pteridine ring, p-aminobenzoic ...
Catalytic triad

A catalytic triad refers to the three amino acid residues that function together at the centre of the active site of some hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, acylases, lipases and β-lactamases). An Acid-Base-Nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine. Because enzymes fold into complex three-dimensional structures, the residues of a catalytic triad can be far from each other along the amino-acid sequence (primary structure), however, they are brought close together in the final fold.As well as divergent evolution of function (and even the triad's nucleophile), catalytic triads show some of the best examples of convergent evolution. Chemical constraints on catalysis have led to the same catalytic solution independently evolving in at least 23 separate superfamilies. Their mechanism of action is consequently one of the best studied in biochemistry.