Section III: A World of Particles
... The atom is composed of even smaller particles called protons, neutrons, and electrons. The protons and neutrons are located in the dense nucleus of the atom. The electrons surround the nucleus. Protons are positively charged, neutrons have no charge, and electrons are negatively charged. Science is ...
... The atom is composed of even smaller particles called protons, neutrons, and electrons. The protons and neutrons are located in the dense nucleus of the atom. The electrons surround the nucleus. Protons are positively charged, neutrons have no charge, and electrons are negatively charged. Science is ...
Chemistry
... Compounds have different properties from the elements of which they are composed. Compounds are represented by chemical formulas that include the symbol for each element followed by a subscript number showing the number of atoms of that element in the compound. (ex. MgCl2) ...
... Compounds have different properties from the elements of which they are composed. Compounds are represented by chemical formulas that include the symbol for each element followed by a subscript number showing the number of atoms of that element in the compound. (ex. MgCl2) ...
effective nuclear charge
... nucleus and repelled by each other outer electrons are shielded from full strength of nucleus ◦ screening effect effective nuclear charge is net positive charge that is attracting a particular electron Z is nuclear charge, S is electrons in lower energy levels ◦ electrons in same energy level contri ...
... nucleus and repelled by each other outer electrons are shielded from full strength of nucleus ◦ screening effect effective nuclear charge is net positive charge that is attracting a particular electron Z is nuclear charge, S is electrons in lower energy levels ◦ electrons in same energy level contri ...
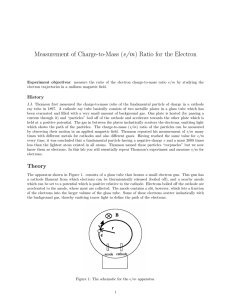
Measurement of Charge-to-Mass (e/m) Ratio for the Electron
... which shows the path of the particles. The charge-to-mass (e/m) ratio of the particles can be measured by observing their motion in an applied magnetic field. Thomson repeated his measurement of e/m many times with different metals for cathodes and also different gases. Having reached the same value ...
... which shows the path of the particles. The charge-to-mass (e/m) ratio of the particles can be measured by observing their motion in an applied magnetic field. Thomson repeated his measurement of e/m many times with different metals for cathodes and also different gases. Having reached the same value ...
Title Building an electron dimer molecule with light Author Massimo
... well as to measure the energy of their excitations. Theoretical calculations have clarified that the motion of the two electrons inside the dot has a vibrational character, being analogous to the one observed for the atoms of a diatomic molecule [1]. Electrons confined in quantum dots are important ...
... well as to measure the energy of their excitations. Theoretical calculations have clarified that the motion of the two electrons inside the dot has a vibrational character, being analogous to the one observed for the atoms of a diatomic molecule [1]. Electrons confined in quantum dots are important ...
File - King`s Senior Science
... positive terminal to the negative terminal. This convention is credited to Benjamin Franklin who theorized that electric current was due to a positive charge moving from the positive terminal to the negative terminal. However, it was later discovered that it is the movement of the negatively charged ...
... positive terminal to the negative terminal. This convention is credited to Benjamin Franklin who theorized that electric current was due to a positive charge moving from the positive terminal to the negative terminal. However, it was later discovered that it is the movement of the negatively charged ...
Electron Multiplying Charge
... important to note that color filters would significantly reduce the quantum efficiency of the device, and most likely would not be preferred in low-light applications. There are two major disadvantages to EMCCD technology. First, it is not possible to quickly gate the device, limiting its high speed ...
... important to note that color filters would significantly reduce the quantum efficiency of the device, and most likely would not be preferred in low-light applications. There are two major disadvantages to EMCCD technology. First, it is not possible to quickly gate the device, limiting its high speed ...
EM SPECTRUM, WAVELENGTH, FREQUENCY, AND ENERGY
... 12. Which scientist proposed the idea that electrons travel around the nucleus in fixed paths? 13. When an electron moves from the ground state to the excited state, energy is ____________________. 14. Bohr chose the element ____________________ to prove his theory. 15. The dual wave-particle nature ...
... 12. Which scientist proposed the idea that electrons travel around the nucleus in fixed paths? 13. When an electron moves from the ground state to the excited state, energy is ____________________. 14. Bohr chose the element ____________________ to prove his theory. 15. The dual wave-particle nature ...
Chapter 5 Notes
... PreAP Chemistry Chapter 5 Notes Section 5.1 The Development of a New Atomic Model Previously, Rutherford reshaped our thought of the atom by showing the protons were located in the _____________________ of the atom, but he could not model for us where the electrons were, other than outside the nucle ...
... PreAP Chemistry Chapter 5 Notes Section 5.1 The Development of a New Atomic Model Previously, Rutherford reshaped our thought of the atom by showing the protons were located in the _____________________ of the atom, but he could not model for us where the electrons were, other than outside the nucle ...
6 Electronic Structure of Atoms
... Stern-Gerlach experiment and line spectra of multi-electron atoms. The SternGerlach experiment shows that a beam of neutral Ag atoms passed through an inhomogeneous magnetic field is deflected equally in two directions. This shows that atoms with a single unpaired electron interact differently with ...
... Stern-Gerlach experiment and line spectra of multi-electron atoms. The SternGerlach experiment shows that a beam of neutral Ag atoms passed through an inhomogeneous magnetic field is deflected equally in two directions. This shows that atoms with a single unpaired electron interact differently with ...
Document
... • A proper understanding of electron and other spin ½ particles came 1928 from theory proposed by Paul Dirac, who for the first time combined both quantum mechanics and special theory of relativity. Thus spin is quantum – relativistic property of the particles. • This theory also predicted that elec ...
... • A proper understanding of electron and other spin ½ particles came 1928 from theory proposed by Paul Dirac, who for the first time combined both quantum mechanics and special theory of relativity. Thus spin is quantum – relativistic property of the particles. • This theory also predicted that elec ...
Electrons!
... Where does this energy come from? Quantum mechanics is a field of physics that answers this. Electrons absorb a specific number of photons of energy when they are excited (heated or absorb some other form of energy). The electrons are not stable in that state and emit photons of energy (in the for ...
... Where does this energy come from? Quantum mechanics is a field of physics that answers this. Electrons absorb a specific number of photons of energy when they are excited (heated or absorb some other form of energy). The electrons are not stable in that state and emit photons of energy (in the for ...
Some Quantum Considerations II
... Which of the following statements is false? a. Longer wavelength radiation carries higher energies. b. Light can be considered to be made up of particles called photons. c. All material objects have some wave characteristics. d. Electrons can be viewed as standing waves in an atom. e. Bohr’s model o ...
... Which of the following statements is false? a. Longer wavelength radiation carries higher energies. b. Light can be considered to be made up of particles called photons. c. All material objects have some wave characteristics. d. Electrons can be viewed as standing waves in an atom. e. Bohr’s model o ...
Chapter 4 Presentation File
... shell can hold 2 electrons. 2nd, 3rd, 4th… can hold 8 electrons. ...
... shell can hold 2 electrons. 2nd, 3rd, 4th… can hold 8 electrons. ...
em spectrum, wavelength, frequency
... 2.) A beam of microwaves has a frequency of 1.0 x 109 Hz. A radar beam has a frequency of 5.0 x 1011 Hz. Which type (microwave or radar)… (A) has a longer wavelength? (B) is closer to visible light on the EM spectrum? (C) is closer to x-rays in frequency value? 3.) What is the frequency of an EM rad ...
... 2.) A beam of microwaves has a frequency of 1.0 x 109 Hz. A radar beam has a frequency of 5.0 x 1011 Hz. Which type (microwave or radar)… (A) has a longer wavelength? (B) is closer to visible light on the EM spectrum? (C) is closer to x-rays in frequency value? 3.) What is the frequency of an EM rad ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.