File
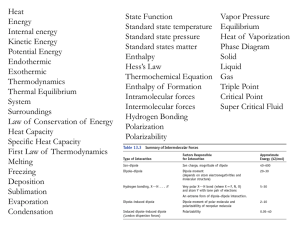
... Gives the order in which atomic orbitals are filled Electrons occupy the orbitals of lowest energy first The Periodic Table is a guide for the Aufbau Principle, ...
... Gives the order in which atomic orbitals are filled Electrons occupy the orbitals of lowest energy first The Periodic Table is a guide for the Aufbau Principle, ...
Frank-Hertz Experiment with Argon
... 1. The electrons can only travel in special orbits: at a certain discrete set of distances from the nucleus with specific energies. 2. The electrons do not continuously lose energy as they travel, which energies are constant. 3. They can only gain and lose energy by jumping from one allowed orbit to ...
... 1. The electrons can only travel in special orbits: at a certain discrete set of distances from the nucleus with specific energies. 2. The electrons do not continuously lose energy as they travel, which energies are constant. 3. They can only gain and lose energy by jumping from one allowed orbit to ...
Honors Chemistry
... 10. Give the different waves of the magnetic spectrum. 11. Which wave has more energy: red or blue? Short or long? Microwave or x-ray? 12. What does Bohr’s Model say about the hydrogen atom? 13. What does it mean when an electron is excited? What happens when the excited electron returns to the grou ...
... 10. Give the different waves of the magnetic spectrum. 11. Which wave has more energy: red or blue? Short or long? Microwave or x-ray? 12. What does Bohr’s Model say about the hydrogen atom? 13. What does it mean when an electron is excited? What happens when the excited electron returns to the grou ...
Topic 7: Atomic and nuclear physics 7.1 The atom
... is somehow excited, then the electrons will leave the ground state and move to a higher energy state. As soon as this happens they transition back to lower energy states radiating energy in the process. The set of wavelengths of light emitted by the atoms of the element is called its emission spectr ...
... is somehow excited, then the electrons will leave the ground state and move to a higher energy state. As soon as this happens they transition back to lower energy states radiating energy in the process. The set of wavelengths of light emitted by the atoms of the element is called its emission spectr ...
QTMN-16.107-166, Layout 1
... The triplet spin state of two electrons with parallel spins is symmetric and appears with the antisymmetric space wavefunction, where the electrons avoid each other (Fermi hole). This is called as spin correlation, which implies lower Coulomb repulsion and lower energy than that of the singlet state ...
... The triplet spin state of two electrons with parallel spins is symmetric and appears with the antisymmetric space wavefunction, where the electrons avoid each other (Fermi hole). This is called as spin correlation, which implies lower Coulomb repulsion and lower energy than that of the singlet state ...
File
... element: a substance that cannot be separated or broken down into simpler substances by chemical means. compound: a substance made up of atoms of two ore more different elements joined by chemical bonds. atomic number: the number of protons in the nucleus of an atom. (The atomic number is the same f ...
... element: a substance that cannot be separated or broken down into simpler substances by chemical means. compound: a substance made up of atoms of two ore more different elements joined by chemical bonds. atomic number: the number of protons in the nucleus of an atom. (The atomic number is the same f ...
Chem 101 notes review
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
video slide
... Atoms -- differ in number of subatomic particles atomic number -- number of protons mass number -- protons + neutrons atomic mass -- approximated by the mass number (mass number + electrons) ...
... Atoms -- differ in number of subatomic particles atomic number -- number of protons mass number -- protons + neutrons atomic mass -- approximated by the mass number (mass number + electrons) ...
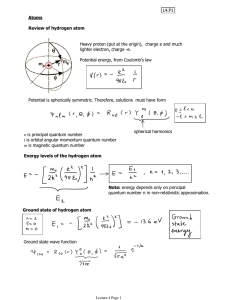
2.4. Quantum Mechanical description of hydrogen atom
... can be called the orbital of the electron. Notes: 1. the energy is quantized; 2. i index denotes that there are several such states. The one with the lowest energy is called the ground state, the others are the excited states. About the solution: during the calculations it turns out that the states ...
... can be called the orbital of the electron. Notes: 1. the energy is quantized; 2. i index denotes that there are several such states. The one with the lowest energy is called the ground state, the others are the excited states. About the solution: during the calculations it turns out that the states ...
ψ 2
... The nucleus is the very dense region consisting of nucleons (protons and neutrons) at the center of an atom. Almost all of the mass in an atom is made up from the protons and neutrons in the nucleus, with a very small contribution from the orbiting electrons. The diameter of the nucleus is in t ...
... The nucleus is the very dense region consisting of nucleons (protons and neutrons) at the center of an atom. Almost all of the mass in an atom is made up from the protons and neutrons in the nucleus, with a very small contribution from the orbiting electrons. The diameter of the nucleus is in t ...
Atoms in Combination: The Chemical Bond
... Calcium and chlorine neutral-atom electron configurations (left), and their configurations after electrons have been transferred from the calcium to the chlorine atoms (right). ...
... Calcium and chlorine neutral-atom electron configurations (left), and their configurations after electrons have been transferred from the calcium to the chlorine atoms (right). ...
Chemistry CP Final Exam Review #2
... Define the following terms: electromagnetic radiation, wavelength, frequency, photons, quantized, line spectrum, continuous spectrum, orbital, principal energy levels, sublevels, electron configuration, orbital diagram, valence electrons, core electrons, representative elements, metals, nonmetals, m ...
... Define the following terms: electromagnetic radiation, wavelength, frequency, photons, quantized, line spectrum, continuous spectrum, orbital, principal energy levels, sublevels, electron configuration, orbital diagram, valence electrons, core electrons, representative elements, metals, nonmetals, m ...
Chapter2. Elements of quantum mechanics
... a. What is the work function of the photocathode surface, in eV? b. If a UV radiation of wavelength 300 nm is incident upon the photocathode surface, what will be the maximum kinetic energy of the photoemitted electrons, in eV? c. Given that the UV light of wavelength 300 nm has an intensity of 20 m ...
... a. What is the work function of the photocathode surface, in eV? b. If a UV radiation of wavelength 300 nm is incident upon the photocathode surface, what will be the maximum kinetic energy of the photoemitted electrons, in eV? c. Given that the UV light of wavelength 300 nm has an intensity of 20 m ...
Atomic Structure
... 1. Principal Quantum Number (n): This has range from 1 to n. This represents the total energy of the system (Do you remember the Bohr’s energy expression). 2. Azimuthul Quantum Number (l): This has range from 0 to n-1. This is related to the orbital angular momemtum. 3. Magnetic Quantum Number (m): ...
... 1. Principal Quantum Number (n): This has range from 1 to n. This represents the total energy of the system (Do you remember the Bohr’s energy expression). 2. Azimuthul Quantum Number (l): This has range from 0 to n-1. This is related to the orbital angular momemtum. 3. Magnetic Quantum Number (m): ...
The Atomic Theory of Matter
... • The rest of the subatomic particles were found when scientists made theories on where the electrons were in an atom. In 1910, a scientist named Rutherford examined the effects of passing alpha rays through a gold foil a few thousand atoms thick. He found that most passed right through the gold foi ...
... • The rest of the subatomic particles were found when scientists made theories on where the electrons were in an atom. In 1910, a scientist named Rutherford examined the effects of passing alpha rays through a gold foil a few thousand atoms thick. He found that most passed right through the gold foi ...
Quantum Numbers
... • Cr should be [Ar]4s23d4 but instead it is [Ar]4s13d5 • Cu should be [Ar]4s23d9 but instead it is [Ar]4s13d10 • These deviations for Cr and Cu are attributed to the particular stability of a half-filled d subshell (the electrons are all the same spin) or a filled d subshells • The other rows of tra ...
... • Cr should be [Ar]4s23d4 but instead it is [Ar]4s13d5 • Cu should be [Ar]4s23d9 but instead it is [Ar]4s13d10 • These deviations for Cr and Cu are attributed to the particular stability of a half-filled d subshell (the electrons are all the same spin) or a filled d subshells • The other rows of tra ...
Bohr model and electron configuration
... Electrons cannot exist between orbits The higher the energy level, the further it is away from the nucleus An atom with maximum number of electrons in the outermost orbital energy level is stable (unreactive) ...
... Electrons cannot exist between orbits The higher the energy level, the further it is away from the nucleus An atom with maximum number of electrons in the outermost orbital energy level is stable (unreactive) ...
Midterm Review
... What is the density of a liquid that has a mass of 50. g and a volume of 300. mL? ...
... What is the density of a liquid that has a mass of 50. g and a volume of 300. mL? ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.