Fluctuation-Induced Forces Between Atoms and
... electromagnetic field (dipole approximation). The factor 1/2 in (11.5) arises from the fact that we are considering the energy of a linearly polarizable particle in an external field, rather than a permanent dipole [43]. Note that the choice of a particular ordering does not seem to be necessary at ...
... electromagnetic field (dipole approximation). The factor 1/2 in (11.5) arises from the fact that we are considering the energy of a linearly polarizable particle in an external field, rather than a permanent dipole [43]. Note that the choice of a particular ordering does not seem to be necessary at ...
The Mole
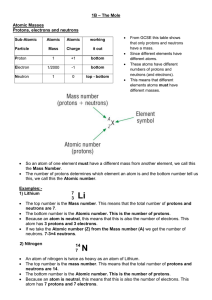
... The top number is the Mass number. This means that the total number of protons and neutrons are 7. The bottom number is the Atomic number. This is the number of protons. Because an atom is neutral, this means that this is also the number of electrons. This atom has 3 protons and 3 electrons. If we t ...
... The top number is the Mass number. This means that the total number of protons and neutrons are 7. The bottom number is the Atomic number. This is the number of protons. Because an atom is neutral, this means that this is also the number of electrons. This atom has 3 protons and 3 electrons. If we t ...
Ground-state stability and criticality of two
... diagram for two-electron atoms with a screened Coulomb potential. The results of this method for two-electron atoms are very accurate in comparison with previous calculations based on Gaussian, Hylleraas and finite-element basis sets. The stability diagram for the screened two-electron atoms shows t ...
... diagram for two-electron atoms with a screened Coulomb potential. The results of this method for two-electron atoms are very accurate in comparison with previous calculations based on Gaussian, Hylleraas and finite-element basis sets. The stability diagram for the screened two-electron atoms shows t ...
Midterm Review
... d. No answer can be determined from the information given. ____ 20. Which variable is directly proportional to frequency? a. wavelength c. position b. velocity d. energy ____ 21. How do the energy differences between the higher energy levels of an atom compare with the energy differences between the ...
... d. No answer can be determined from the information given. ____ 20. Which variable is directly proportional to frequency? a. wavelength c. position b. velocity d. energy ____ 21. How do the energy differences between the higher energy levels of an atom compare with the energy differences between the ...
Physics 139B Solutions to Homework Set 4 Fall 2009 1. Liboff
... probability of a transition from the ground state to the first excited state is nonnegligible. However, keep in mind that if P0→1 must still be small as compared to 1 if the perturbation theory result is to be reliable. In case (b), there are no first-order transitions from the ground state to excit ...
... probability of a transition from the ground state to the first excited state is nonnegligible. However, keep in mind that if P0→1 must still be small as compared to 1 if the perturbation theory result is to be reliable. In case (b), there are no first-order transitions from the ground state to excit ...
Multimode quantum memory based on atomic frequency combs
... although the atomic frequency comb has large “holes.” This can be understood by considering that the absorption is an event well localized in time, of the order = 1 / ␥ p. In that case the light-atom system reacts with a Fourier-limited resolution ␥ p, which effectively smears out the structure of ...
... although the atomic frequency comb has large “holes.” This can be understood by considering that the absorption is an event well localized in time, of the order = 1 / ␥ p. In that case the light-atom system reacts with a Fourier-limited resolution ␥ p, which effectively smears out the structure of ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.