“Nature is made in such a way as to be able to be understood
... Copenhagen Interpretation... Bohr, Heisenberg.. ...
... Copenhagen Interpretation... Bohr, Heisenberg.. ...
The Atom
... • Emission of positively charged particles from radioactive elements was somewhat clear already. A radioactive element served as Rutherford’s source of alpha particles. ...
... • Emission of positively charged particles from radioactive elements was somewhat clear already. A radioactive element served as Rutherford’s source of alpha particles. ...
File - Ingolstadt Academy
... Accuracy and Precision Significant Figures and Uncertainty Scientific notation Metric System Using proper units/labels Density (definition and equation) Dimensional analysis Instruments that measure mass, volume, pressure, etc. (lab stuff!) The Scientific Method Atomic Structure: ...
... Accuracy and Precision Significant Figures and Uncertainty Scientific notation Metric System Using proper units/labels Density (definition and equation) Dimensional analysis Instruments that measure mass, volume, pressure, etc. (lab stuff!) The Scientific Method Atomic Structure: ...
Materials Science for Chemical Engineers
... The Electronic Structure of the Atom Electrons occupy discrete energy levels within the atom. The energy level to which each electron belongs is determined by 4 quantum numbers: The principal quantum number n (1, 2, 3, …) The azimuthal quantum number l The magnetic quantum number ml The spin quantu ...
... The Electronic Structure of the Atom Electrons occupy discrete energy levels within the atom. The energy level to which each electron belongs is determined by 4 quantum numbers: The principal quantum number n (1, 2, 3, …) The azimuthal quantum number l The magnetic quantum number ml The spin quantu ...
Atomic Orbitals
... Valence electrons – electrons in the outermost (highest) principal energy level of an atom Core electrons – inner electrons Elements with the same valence electron arrangement show very similar chemical behavior. ...
... Valence electrons – electrons in the outermost (highest) principal energy level of an atom Core electrons – inner electrons Elements with the same valence electron arrangement show very similar chemical behavior. ...
File - Mrs. Hille`s FunZone
... Further they fall, more energy, higher frequency. This is simplified the orbitals also have different energies inside energy levels All the electrons can move around. ...
... Further they fall, more energy, higher frequency. This is simplified the orbitals also have different energies inside energy levels All the electrons can move around. ...
Ch.41- Orbital angular momentum, counting states
... momentum has the same magnitude L for each these five states, but has different values of the zcomponent Lz. Copyright © 2012 Pearson Education Inc. ...
... momentum has the same magnitude L for each these five states, but has different values of the zcomponent Lz. Copyright © 2012 Pearson Education Inc. ...
Atomic Theory MC 2012
... 11. Which of the following is a correct interpretation of the results of Rutherford's experiments in which gold atoms were bombarded with alpha particles? (A) Atoms have equal numbers of positive and negative charges. (B) Electrons in atoms are arranged in shells. (C) Neutrons are at the center of a ...
... 11. Which of the following is a correct interpretation of the results of Rutherford's experiments in which gold atoms were bombarded with alpha particles? (A) Atoms have equal numbers of positive and negative charges. (B) Electrons in atoms are arranged in shells. (C) Neutrons are at the center of a ...
General Chemistry: An Integrated Approach
... • The atom has gone through some changes, where are we now? • 1. Democritus/Dalton = small, spheres. • 2. Thomson = plum pudding model. • 3. Rutherford = planetary model. • The model is incomplete – it didn’t really explain where electrons were outside the nucleus. ...
... • The atom has gone through some changes, where are we now? • 1. Democritus/Dalton = small, spheres. • 2. Thomson = plum pudding model. • 3. Rutherford = planetary model. • The model is incomplete – it didn’t really explain where electrons were outside the nucleus. ...
2. Fermi Statistics of Electrons and Some Definitions
... with the vectors k, and the components kx , ky , kz have to be interpreted as quantum numbers, up to now noted as index j in (#j , ϕj ): The state of an electron of the Hamilton operator (2.17) is labeled by the quantum number k and the spin s. The wave length λ = 2π/k ...
... with the vectors k, and the components kx , ky , kz have to be interpreted as quantum numbers, up to now noted as index j in (#j , ϕj ): The state of an electron of the Hamilton operator (2.17) is labeled by the quantum number k and the spin s. The wave length λ = 2π/k ...
Chemistry 102B What`s in an atom? Before “Chemistry” Other Early
... Developed the “Law of conservation of Mass”. • Joseph Proust (early 1800s) – discovered that a given compound always contained the same proportions of certain elements by mass. “Law of Definite Proportions” • John Dalton (early 1800s) – noted that elements that combined to form more than one kind of ...
... Developed the “Law of conservation of Mass”. • Joseph Proust (early 1800s) – discovered that a given compound always contained the same proportions of certain elements by mass. “Law of Definite Proportions” • John Dalton (early 1800s) – noted that elements that combined to form more than one kind of ...
CHAPTER 10 - NUCLEAR PHYSICS
... For most elements this is eight electrons. For hydrogen, lithium, beryllium, and boron this number is two since they reach their lowest energy state with an electron configuration like helium. In a 100% ionic bond, an electron is transferred from one atom to another making the atom that lost the ele ...
... For most elements this is eight electrons. For hydrogen, lithium, beryllium, and boron this number is two since they reach their lowest energy state with an electron configuration like helium. In a 100% ionic bond, an electron is transferred from one atom to another making the atom that lost the ele ...
CCR 19: Spectroscopic Notation
... (P term) state, the same limiting term as for the sharp series. Finally, the fundamental emission spectrum was the result of transitions from the higher fundamental (F terms) energy states to the lowest D term state, the limiting term for the fundamental series. In Figure SN-3 the principal emission ...
... (P term) state, the same limiting term as for the sharp series. Finally, the fundamental emission spectrum was the result of transitions from the higher fundamental (F terms) energy states to the lowest D term state, the limiting term for the fundamental series. In Figure SN-3 the principal emission ...
chemia simr01 en - Leszek Niedzicki
... • Small hydrogen without its electron has positive charge accumulated in a small volume (not distributed on any neutrons); • In molecules in which hydrogen gives his electron away to atoms with strong affinity towards electrons (e.g. oxygen, nitrogen, fluorine) its electron (although formally shared ...
... • Small hydrogen without its electron has positive charge accumulated in a small volume (not distributed on any neutrons); • In molecules in which hydrogen gives his electron away to atoms with strong affinity towards electrons (e.g. oxygen, nitrogen, fluorine) its electron (although formally shared ...
LAMB SHIFT & VACUUM POLARIZATION CORRECTIONS TO THE
... tion, Dirac devised a relativistic wave equation that is linear in both ∂/∂t and ∇, although he succeeded in avoiding the negative probability density, negative-energy solutions still occurred. That means that an atomic electron can have both negative and positive energies. But according to the qua ...
... tion, Dirac devised a relativistic wave equation that is linear in both ∂/∂t and ∇, although he succeeded in avoiding the negative probability density, negative-energy solutions still occurred. That means that an atomic electron can have both negative and positive energies. But according to the qua ...
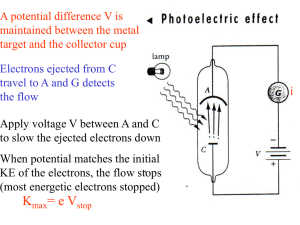
V stop f
... • average KE ~ amplitude2 ~(electric field)2~Intensity • Quantum theory: • light composed of energy packets called photons E=hf =>an electron absorbs one photon and gains energy hf (this process is independent of the intensity) • not expected classically! => increase intensity or wait longer for ele ...
... • average KE ~ amplitude2 ~(electric field)2~Intensity • Quantum theory: • light composed of energy packets called photons E=hf =>an electron absorbs one photon and gains energy hf (this process is independent of the intensity) • not expected classically! => increase intensity or wait longer for ele ...
Population_analysis_Ranjit
... molecules into atoms called the Quantum Theory of Atoms in Molecules (QTAIM). His definition of an atom is based purely on the electronic charge density. Bader uses what are called zero flux surfaces to divide atoms. A zero flux surface is a 2-D surface on which the charge density is a minimum perpe ...
... molecules into atoms called the Quantum Theory of Atoms in Molecules (QTAIM). His definition of an atom is based purely on the electronic charge density. Bader uses what are called zero flux surfaces to divide atoms. A zero flux surface is a 2-D surface on which the charge density is a minimum perpe ...
quantum-theory-of-the-atom2
... of both a particle and a wave, we can start to understand the emission spectra of atoms. One in particular, hydrogen (shown below) ...
... of both a particle and a wave, we can start to understand the emission spectra of atoms. One in particular, hydrogen (shown below) ...
Introduction to Quantum Mechanics: Homework #1 (Due by Sep
... 6. Consider a grey body whose emissivity ε (λ) = 0.6 at all λ. How much energy is emitted from the grey body at T =1500 K per unit surface area in 1 sec? 7. Based on the Einstein’s model for the heat capacity Cv of a solid, show that 1) Cv→0 as T→0 and 2) Cv = 3R at high T. 8. Suppose one performs p ...
... 6. Consider a grey body whose emissivity ε (λ) = 0.6 at all λ. How much energy is emitted from the grey body at T =1500 K per unit surface area in 1 sec? 7. Based on the Einstein’s model for the heat capacity Cv of a solid, show that 1) Cv→0 as T→0 and 2) Cv = 3R at high T. 8. Suppose one performs p ...
3 Nov 08 - Seattle Central College
... • We can model the attraction of the H atom’s single electron to its single proton with a “Coulombic” potential curve: ...
... • We can model the attraction of the H atom’s single electron to its single proton with a “Coulombic” potential curve: ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.